Nitrite Formula
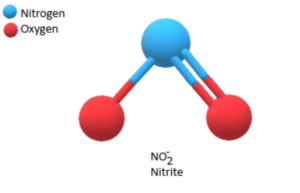
The formula for nitrite is NO₂⁻, indicating a negatively charged polyatomic ion composed of one nitrogen atom (N) and two oxygen atoms (O).
Formula and Structure of Nitrite
In the nitrite ion (NO₂⁻), the nitrogen atom is bonded to the two oxygen atoms through covalent bonds. The overall charge of -1 is achieved by the nitrogen atom having a lone pair of electrons, which contributes to the negative charge of the ion.
Physical Properties of Nitrite Formula
- Charge: The nitrite ion carries a charge of -1.
- Molecular Weight: The molecular weight of nitrite is approximately 46.01 grams per mole.
- State of Matter: Nitrite ions can exist as solids or dissolved in solution.
- Solubility: Nitrites are generally soluble in water and other polar solvents.
- Color: Pure nitrite compounds are typically colorless or pale yellow.
Also Check: Zinc Nitrate Formula
Chemical Properties of Nitrite Formula
- Oxidation-Reduction Reactions: Nitrites can undergo oxidation-reduction reactions, serving as either oxidizing agents or reducing agents, depending on the reaction conditions and other substances involved.
- Acid-Base Reactions: Nitrite ions can act as weak bases and form salts with strong acids.
- Formation of Nitrous Acid: Nitrite ions can react with acids to form nitrous acid (HNO₂), which can further decompose into nitrogen dioxide (NO₂) and water (H₂O).
Solved Examples of Nitrite Formula
Example 1: Oxidizing Agent
In a chemical reaction, nitrite ions (NO₂⁻) can act as oxidizing agents. For example, when nitrite reacts with iodide ions (I⁻) in an acidic solution, the nitrite gets reduced to nitrogen monoxide (NO), while iodide gets oxidized to iodine (I₂).
2 NO₂⁻ + 4 H⁺ + 2 I⁻ → 2 NO + 2 H₂O + I₂
In this reaction, nitrite is serving as an oxidizing agent by accepting electrons from iodide ions and undergoing reduction.
Example 2: Formation of Nitrous Acid
Answer: When nitrite ions (NO₂⁻) react with water (H₂O) in an acidic environment, they form nitrous acid (HNO₂).
NO₂⁻ + H₂O → HNO₂
The nitrite ion acts as a weak base in this reaction, accepting a proton from water and forming nitrous acid.
Frequently asked questions on Nitrite
1: What is nitrite used for?
Answer: In food processing and biochemistry, nitrite. Sodium nitrite is used to treat foods, as it inhibits bacterial growth and explicitly inhibits botulism. Nitrite inhibits the germination of endospores of C.
2: Why is nitrite toxic?
Answer: Bacteria in our saliva, stomach, and intestines convert nitrates into nitrites and it is primarily the nitrites that cause toxicity. The iron component of red blood cells (haemoglobin) is oxidized by nitrites making them unable to carry oxygen.
3: What is the difference between nitrate and nitrite?
Answer: The distinction between the two chemicals is that they contain oxygen. Nitrates are composed of 3 oxygen atoms with a chemical NO3 formula, whereas Nitrites have 2 oxygen atoms that make up a chemical NO2 formula. Nitrites are converted and made into healthy chemical Nitric Oxide.
4: What is the ion of nitrite?
Answer: Nitrite is an ion consisting of an atom of nitrogen bound up with two oxygen atoms. The nitrite is actually known as an anion. An ion is a positively or negatively charged molecule, and an anion is a type of ion bearing a negative charge. Nitrite’s molecular Weight is 46.01g / mol.
5: What does nitrite smell like?
Answer: Amyl nitrite is an extremely flammable, highly volatile, clear or yellow-colored oil that is commonly inhaled from a small glass bottle. It typically has a distinct smell that resembles dirty socks.




