Chemistry Practical Class 12 Thermochemistry
Enthalpy of Dissolution
It is well known that when a solute is dissolved in a solvent, heat is either absorbed or evolved. Thus, dissolution of a solute in a solvent is accompanied by enthalpy change (∆H) of the system. If heat is absorbed (i.e., the solution gets cooled), ∆H is given a positive sign. If heat is evolved (i.e., the solution gets warmed), ∆H is given negative sign.
The enthalpy change per mole of a solute dissolved varies with the concentration of the solution. Therefore, it is necessary to express the enthalpy change with reference to the concentration of the solution. The enthalpy of dissolution is defined as the enthalpy change per mole of a solute when it is dissolved in a pure solvent to give a solution of specified concentration. For example, when one mole of anhydrous calcium chloride is dissolved in 400 moles of water, 78.60 kJ heat is evolved. A thermochemical equation to express this can be written as under:
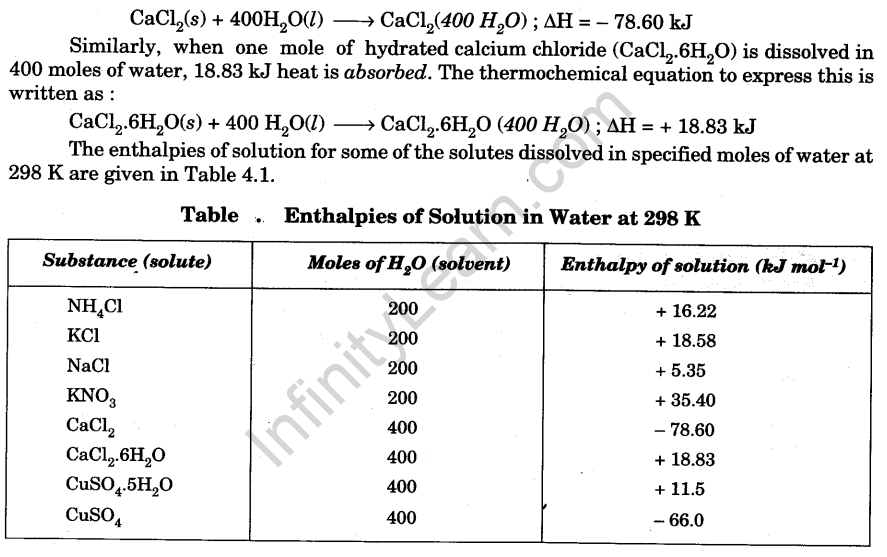
Enthalpy of Neutralisation
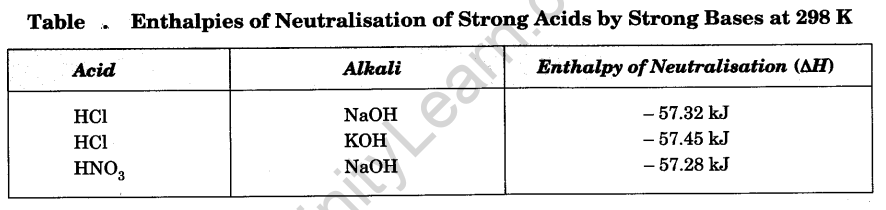
Enthalpy of neutralisation of an acid at a given temperature is defined as enthalpy change (∆H) accompanying the neutralisation of one gram equivalent of the acid by a base in dilute solutions at that temperature. Enthalpy of neutralisation of an acid may also be defined as the enthalpy change accompanying the formation of one mole of water by reaction between the acid and a base in dilute solutions. The neutralisation of hydrochloric acid by sodium hydroxide in dilute solutions at 298 K is represented by the thermochemical equation.
HCl (aq) + NaOH (aq) ——–> NaCl (aq) + H20 (l); ∆H = – 57.32 kJ
Thus, the enthalpy of neutralisation of hydrochloric acid by sodium hydroxide at 298 K is – 57.32 kJ.
Similarly, enthalpy change accompanying neutralisation of one gram equivalent of a base by an acid in dilute solutions at a given temperature is known as the enthalpy of neutralisation of the base at that temperature. In the above example, the enthalpy of neutralisation of sodium hydroxide with hydrochloric acid is also – 57.32 kJ at 298 K.
The neutralisation of hydrochloric acid by sodium hydroxide in dilute solutions, when the acid, alkali and the salt formed are completely dissociated, may be represented as
H+ (aq) + Cl– (aq) + Na+ (aq) + OH– (aq) ——–> Na+ (aq) + Cl– (aq) + H2O(l)
or H+ (aq) + OH– (aq) ——–>H20(l)
Neutralisation of strong acid and strong base involves the combination of H+ and OH– ions to form unionised water. It is, therefore, expected that the enthalpy of neutralisation of every strong acid by a strong base should be same and this is largely so, as is evident from the data in Table .
Calorimeter Constant of Calorimeter
A calorimeter is an apparatus used to measure the heat evolved or absorbed in a chemical or physical process. The calorimeter constant is a measure of the accuracy of the calorimeter. The calorimeter constant is the heat capacity of the calorimeter divided by the mass of the calorimeter. The calorimeter constant can be determined by experiment.




