Table of Contents
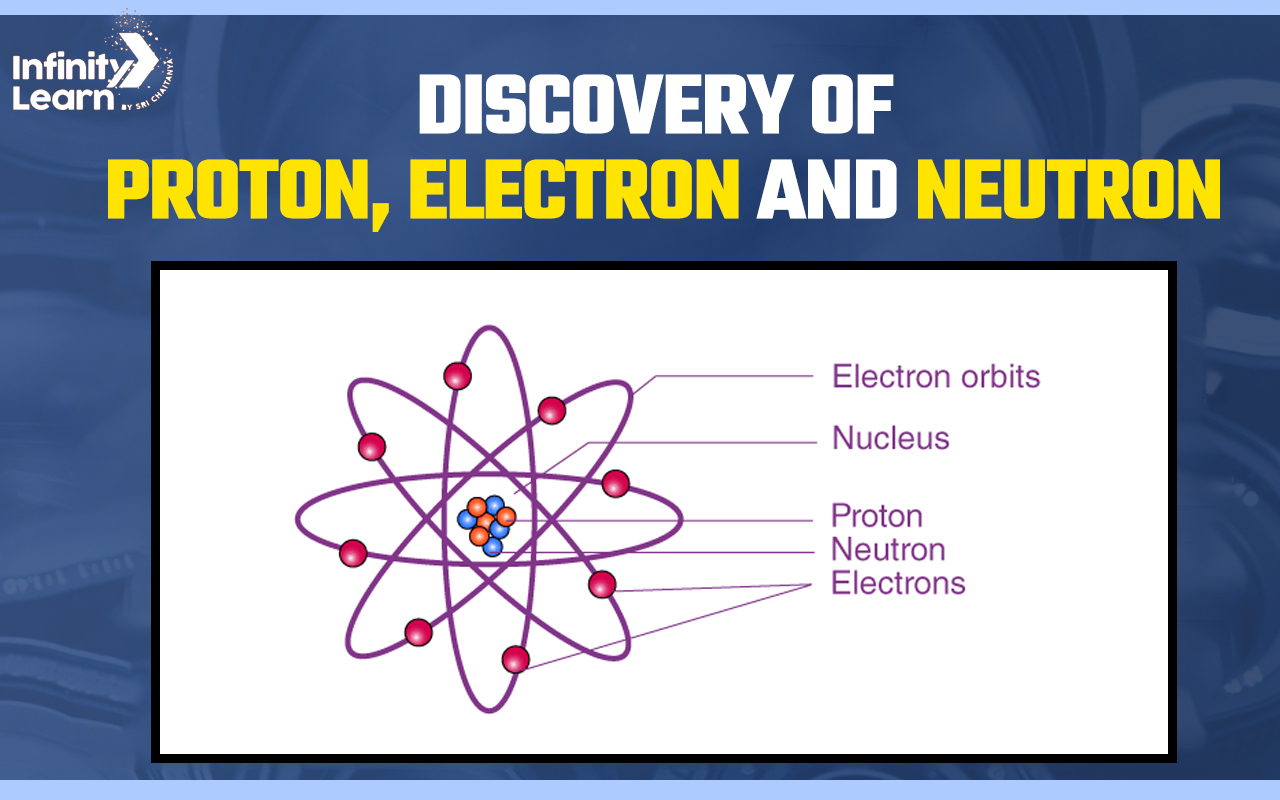
Atom is the smallest unit of matter comprising three subatomic particles: protons, neutrons and electrons. These three subatomic particles, protons, electrons and neutrons, were identified during the nineteenth and twentieth centuries. Matter (solids, liquids and gasses) are formed by molecules, and these molecules are made up of atoms.
Who Discovered The Proton?
Protons were discovered by Ernest Rutherford in 1909 while performing a gold foil experiment.
Rutherford conducted his famous alpha scattering experiment, the first definitive experiment to establish the basic structure of atoms, especially the discovery of nuclei containing protons and neutrons.
This Rutherford Model of the atom was also known as the “Rutherford Nuclear Atom”.
This model was also known as the “Rutherford Planetary Model” because it was similar to the solar system, with the nucleus representing the sun and revolving electrons as planets.
He also suggested that the electrons circle the nucleus at the speed of light.
- To determine how electrons are arranged in an atom, the alpha particle scattering was organised by Rutherford.
- Rutherford bombarded a thin sheet of gold by fast-moving alpha particles coming from a radioactive source and observed their deviations after passing through the gold foil.
- A fluorescent zinc sulfide screen was also placed around the thin gold foil so that he could study the deflection caused by the alpha particles.
How was Proton Discovered?
Ernest Rutherford discovered the proton through simple yet impactful experiments. When he shot alpha particles into the air, detectors found hydrogen nuclei. Investigating further, he realized these came from nitrogen atoms in the air. Firing alpha particles into pure nitrogen created even more hydrogen nuclei. Rutherford concluded that all atoms had hydrogen nuclei.
This marked the first nuclear reaction, expressed as
14N + alpha -> 17O + p
where is an alpha particle (with two protons and two neutrons) and ‘p’ is a proton.
The hydrogen nucleus was later named ‘proton,’ becoming a crucial building block in our understanding of atoms.
Observations of Rutherford’s Alpha Scattering Experiment
The following observations were made-
- Most of the particles pass through the gold foil with little or no deviation, meaning most of the space inside an atom is empty.
- Very few particles went through a deviation of about 5°, so there must be some positively charged region in the atom.
- Even fewer particles (1/20000) suffered a deflection of 90° or more and even a complete rebound, so the positively charged region was very small compared to the atom.
Based on observations, Rutherford proposed the following atomic model:
- The positive charge of the atom is concentrated in a very small space called “The Nucleus”. The positively charged particle is called a proton.
- Most of the space is empty in an atom.
- A strong electrostatic force of attraction holds the nucleus together as the electrons are negatively charged and, nucleus is made up of positively charged particles.
Properties of Protons
- Protons are positively charged.
- Protons travel in straight lines.
- Both electric and magnetic fields deflect these positive rays
- Mass of the proton is 1.672 x 10-24 g.
- The charge on the proton is +1.602 x 10-19 coulombs.
- The volume of a proton is given by 4/3 πr3 (1.5 x 10-38 cm3)
Who discovered the Neutrons
Another subatomic particle of an atom is the neutron. The charge on a neutron is zero. These particles were discovered by Chadwick in 1932. Hydrogen atoms do not contain neutrons, else all the atoms contain neutrons.
- James Chadwick utilised a polonium source to emit alpha radiation at a beryllium sheet. As a result, uncharged penetrating radiation was generated.
- This radiation was incident on paraffin wax, a hydrocarbon with a high hydrogen content.
- Protons expelled from paraffin wax (when touched by uncharged radiation) were seen using an ionisation chamber.
- Chadwick investigated the interaction of uncharged radiation with the atoms of several gasses and determined the range of liberated protons.
- He concluded that the exceptionally penetrating radiation was composed of uncharged particles, the mass of a proton. These particles later received neutrons.
Properties of Neutrons
- Protons are neutral particles.
- The mass of neutrons and protons are equal (the Mass of the neutron is 1.675 x 10-24 g).
- The specific charge of a neutron is zero.
- The density of the neutron is 1.5 x 1014 g/cc.
Who Discovered the Electrons
Electrons are also subatomic particles of an atom which are negatively charged. Electrons were discovered by JJ Thomson in 1897 in a cathode ray experiment.
Discovery of electrons
In a cathode ray experiment, when the pressure of a discharge tube maintained at high potential is reduced, a stream of negatively charged particles originates from cathode known as electrons.
These originated rays are cathode rays. These rays themselves are not visible but their behavior can be observed with the help of fluorescent or phosphorescent materials.
These rays travel in a straight line and are deflected by electrical and magnetic fields the same way as electrons.
Properties of electrons
- The charge on an electron is 1.60217663 × 10-19 coulombs.
- Mass of an electron is 9.11×10-31 kg
- Electrons are the lightest among the three fundamental particles of atoms.
FAQs on Discovery of Proton, Electron & Neutron
What is a proton?
A proton is a subatomic particle of an atom. It has a positive charge in it
Who discovered neutrons?
Neutrons were discovered by James Chadwick. He was also awarded the Nobel Prize in 1935 for his work.
What are the three subatomic particles present in an atom?
The three subatomic particles present in an atom are electrons, protons and neutrons.








