Salicylic Acid Formula
Salicylic acid is a white crystalline organic compound with the chemical formula C₇H₆O₃. It is derived from the bark of willow trees and is widely used in various skincare products and pharmaceuticals. Salicylic acid possesses keratolytic properties, making it effective in exfoliating the skin and unclogging pores.
It is commonly used to treat acne, warts, and other skin conditions. Additionally, salicylic acid is a key precursor in the synthesis of aspirin (acetylsalicylic acid) and other pharmaceutical compounds. Its acidic nature allows it to act as a mild exfoliant and help remove dead skin cells, promoting clearer and healthier-looking skin.
Formula and Structure of Salicylic Acid:
In salicylic acid, the molecular structure consists of a benzene ring with a carboxyl group (-COOH) attached at position 1 and a hydroxyl group (-OH) attached at position 2. The structure can be represented as follows:
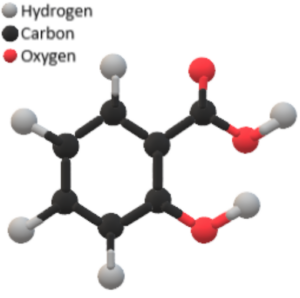
Structure of Salicylic Acid
Physical Properties of Salicylic Acid:
- State of Matter: Salicylic acid is a white crystalline solid.
- Melting Point: The melting point of salicylic acid is approximately 159 degrees Celsius (318 degrees Fahrenheit).
- Solubility: Salicylic acid is sparingly soluble in water but dissolves readily in organic solvents like ethanol and ether.
- Odor: Salicylic acid has a slight acetic acid-like odor.
Chemical Properties of Salicylic Acid:
- Acidic Nature: Salicylic acid is a weak acid due to the presence of the carboxyl group. It can donate a proton (H⁺) when dissolved in water, resulting in the formation of the salicylate ion (C₇H₅O₃⁻).
- Hydroxylation Reactions: Salicylic acid can undergo hydroxylation reactions, where the hydroxyl group is modified or substituted by other chemical groups.
- Esterification: Salicylic acid can react with alcohols to form esters, a process commonly used in the synthesis of salicylates, such as methyl salicylate.
- Keratolytic Action: Salicylic acid has a keratolytic effect, meaning it can help exfoliate and remove dead skin cells. This property makes it useful in skincare products and treatments for acne, warts, and other skin conditions.
Solved Examples on Salicylic Acid:
Example 1: Synthesis of Aspirin
Answer: Salicylic acid is a key starting material in the synthesis of aspirin (acetylsalicylic acid). In this process, salicylic acid reacts with acetic anhydride in the presence of a catalyst, such as sulfuric acid, to form aspirin and acetic acid.
C₇H₆O₃ + C₄H₆O₃ → C₉H₈O₄ + CH₃COOH
In this reaction, the hydroxyl group of salicylic acid reacts with acetic anhydride to form an ester, resulting in the production of aspirin.
Example 2: Treatment of Acne
Answer: Salicylic acid is used as an active ingredient in many acne treatment products. It helps to exfoliate the skin and unclog pores by removing dead skin cells and reducing sebum production. The salicylic acid penetrates the skin and can help in treating and preventing acne breakouts.
Frequently asked questions on Salicylic Acid
1: What are the uses of salicylic acid?
Answer: Salicylic acid acts as a keratolytic (it serves as a peeling agent). Salicylic acid facilitates and makes the outer layer of the skin shed. The topical salicylic acid (for the skin) is used to treat acne, dandruff, seborrhea, or psoriasis and to remove cotton, calluses, and warts. Salicylic acid is found in many daily-use products.
2: What does salicylic acid do to your skin?
Answer: Salicylic acid works by loosening and breaking apart desmosomes in the outer layers of the skin, which are attachments between cells. This action helps the skin to exfoliate and the pores to unclog. Salicylic acid is capable of reducing sebum secretion, which is another way to help reduce acne.
3: Is salicylic acid safe?
Answer: Although the use of low-concentration household salicylic acid products is usually considered safe, salicylic acid can cause mild chemical burns at high concentrations. These chemicals can also cause dangerous intoxication if ingested.
4: What is the old name of salicylic acid?
Answer: The old name of salicylic acid is derived from its natural source, willow bark. It was historically known as “spirits of salicylic acid” or simply “salicylic acid.” The name “salicylic acid” itself comes from the Latin word “salix,” which means willow tree. This name reflects the origin of salicylic acid from the bark of willow trees, which has been used for centuries in traditional medicine to relieve pain and reduce fever.
5: What are the important points of salicylic acid?
Answer: The some important points about salicylic acid:
Chemical Composition: Salicylic acid has the chemical formula C₇H₆O₃. It is a monohydroxybenzoic acid derived from the benzene ring structure.
Natural and Synthetic Sources: Salicylic acid can be derived from natural sources, such as the bark of willow trees, or it can be synthesized chemically.
Skincare Applications: Salicylic acid is widely used in skincare products for its keratolytic properties. It helps to exfoliate the skin, unclog pores, and treat acne, blackheads, and other skin conditions.
Anti-inflammatory and Analgesic Effects: Salicylic acid has mild anti-inflammatory and analgesic (pain-relieving) properties, which have been utilized in topical treatments for joint pain, muscle soreness, and minor skin irritations.
Aspirin Synthesis: Salicylic acid is a key starting material in the synthesis of acetylsalicylic acid, commonly known as aspirin. By acetylating salicylic acid, aspirin is produced, which exhibits analgesic, antipyretic (fever-reducing), and anti-inflammatory effects.
Mild Acidic Nature: Salicylic acid is a weak acid with a pKa value of approximately 2.97. Its mild acidity contributes to its exfoliating and peeling properties, as well as its ability to dissolve certain types of skin debris.
Caution with High Concentrations: While lower concentrations of salicylic acid are generally safe for topical use, high concentrations or prolonged exposure can cause skin irritation, dryness, and peeling. It is important to follow usage instructions and consult a healthcare professional if needed.
Professional and Medical Applications: Salicylic acid is used by professionals, such as dermatologists, in higher concentrations for chemical peels and other cosmetic procedures. It is also utilized in various medical treatments, including wart removal and scalp conditions like dandruff.




