Sodium hydroxide (NaOH) is a chemical compound consisting of one sodium ion (Na+) and one hydroxide ion (OH–). It is commonly known as caustic soda and is a strong base. Sodium hydroxide is highly soluble in water and has a wide range of applications in various industries.
Chemical Formula: The chemical formula of sodium hydroxide is NaOH. The subscript numbers indicate the ratio of the ions present in the compound, with one sodium ion (Na+) and one hydroxide ion (OH–).
Definition: Sodium hydroxide is an inorganic compound that is considered a strong base. It is highly caustic and has a slippery feel. It is commonly used in chemical processes, manufacturing, cleaning agents, and in the production of various chemicals.
Equations involving Sodium Hydroxide
- Dissociation in Water: When sodium hydroxide is dissolved in water, it dissociates into sodium ions (Na+) and hydroxide ions (OH–). The equation for this dissociation is: NaOH → Na+ + OH–
- Neutralization Reaction: Sodium hydroxide reacts with acids in a neutralization reaction to form water and salt. The equation for a generic neutralization reaction with hydrochloric acid (HCl) is: NaOH + HCl → NaCl + H2O
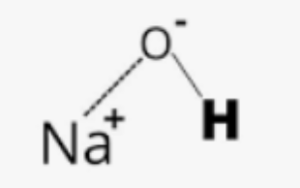
Structural Formula of Sodium Hydroxide:
Sodium hydroxide is an ionic compound, and as such, it does not have a distinct structural formula like covalent compounds. It is represented by the chemical formula NaOH, which indicates the arrangement of sodium ions and hydroxide ions in the compound. The sodium ion (Na+) and hydroxide ion (OH–) are held together by ionic bonds.
The structural formula of sodium hydroxide can be represented as:
This structure illustrates the connection between the sodium ion (Na+) and the hydroxide ion (OH–) in the compound.
Sodium hydroxide is a highly corrosive substance and should be handled with care. It is important to follow proper safety precautions when working with sodium hydroxide, such as wearing protective gloves, goggles, and working in a well-ventilated area.
Physical properties of Sodium Hydroxide:
- State: Caustic soda is typically found in solid form at room temperature. It appears as white, crystalline flakes, pellets, or granules. However, it can also be obtained as a concentrated aqueous solution.
- Melting Point: The melting point of caustic soda is approximately 318°C (604°F). At this temperature, solid sodium hydroxide undergoes a phase change and becomes a liquid.
- Solubility: Sodium hydroxide is highly soluble in water, and its solubility increases with temperature. It readily dissolves in water, releasing hydroxide ions (OH–) and sodium ions (Na+). The resulting solution is strongly alkaline.
- Odour: Caustic soda does not possess a characteristic odor. However, its solution can give off a soapy or slippery sensation when touched due to its alkaline nature.
- Density: The density of sodium hydroxide varies depending on its concentration and temperature. Solid caustic soda has a higher density than water, while its aqueous solutions have densities less than pure water.
- Hygroscopicity: Sodium hydroxide is hygroscopic, meaning it can absorb moisture from the air. It readily attracts water molecules, which can lead to the formation of a concentrated solution when exposed to humid conditions.
- Corrosiveness: Caustic soda is highly corrosive to various materials, including metals, organic tissues, and certain plastics. It can cause severe burns and tissue damage upon direct contact. Proper precautions must be taken when handling and storing sodium hydroxide to avoid accidents.
Chemical properties of Sodium Hydroxide:
- Strong Base: Sodium hydroxide is a strong base and dissociates in water to release hydroxide ions (OH–). It readily reacts with acids in neutralization reactions, forming water and a corresponding salt.
- Neutralization Reactions: Caustic soda reacts with acids, such as hydrochloric acid (HCl), sulfuric acid (H2SO4), or acetic acid (CH3COOH), to produce water and the respective salt.
For example: NaOH + HCl → NaCl + H2O
NaOH + H2SO4 → Na2SO4 + H2O
NaOH + CH3COOH → CH3COONa + H2O
- Alkaline Nature: Sodium hydroxide solutions are highly alkaline due to the presence of hydroxide ions. They have a high pH value and can cause skin and eye irritation upon contact.
- Reactivity with Metals: Sodium hydroxide reacts with certain metals, such as aluminum, zinc, and tin, to produce hydrogen gas and the corresponding metal hydroxide. For example: 2NaOH + 2Al → 2 NaAlO2 + H2↑
- Reactivity with Amphoteric Substances: Sodium hydroxide can react with amphoteric substances, which can act as both acids and bases. This reaction results in the formation of salts. For example, sodium hydroxide reacts with aluminum oxide (Al2O3) to form sodium aluminate (NaAlO2): 2 NaOH + Al2O3 → 2NaAlO2 + H2O
- Saponification: Sodium hydroxide is used in the process of saponification, where it reacts with fats or oils to produce soap. The hydroxide ions break down the ester bonds in the fats or oils, resulting in the formation of soap molecules and glycerol.
- Dehydration: Sodium hydroxide is capable of dehydrating certain compounds by removing water molecules. It can react with alcohols to form alkoxides and water. For example: NaOH + C2H5OH → C2H5ONa + H2O
Solved Examples on Sodium hydroxide formula:
Example 1: Calculation of Molar Mass Calculate the molar mass of sodium hydroxide (NaOH).
The molar mass of sodium is 22.99 g/mol, the molar mass of oxygen is 16.00 g/mol, and the molar mass of hydrogen is 1.01 g/mol.
To calculate the molar mass of sodium hydroxide (NaOH), we add the molar masses of sodium, oxygen, and hydrogen.
Molar Mass = (Number of sodium atoms × Molar mass of sodium) + (Number of oxygen atoms × Molar mass of oxygen) + (Number of hydrogen atoms × Molar mass of hydrogen)
Molar Mass = (1 × 22.99 g/mol) + (1 × 16.00 g/mol) + (1 × 1.01 g/mol)
Molar Mass = 22.99 g/mol + 16.00 g/mol + 1.01 g/mol
Molar Mass = 40.00 g/mol
Therefore, the molar mass of sodium hydroxide (NaOH) is 40.00 g/mol.
Example 2: Stoichiometry in a Reaction Sodium hydroxide (NaOH) reacts with hydrochloric acid (HCl) to form sodium chloride (NaCl) and water (H2O). If you mix 10 grams of sodium hydroxide with excess hydrochloric acid, what is the theoretical yield of sodium chloride?
The balanced chemical equation for the reaction is:
NaOH + HCl → NaCl + H2O
According to the stoichiometry of the balanced equation, 1 mole of sodium hydroxide reacts with 1 mole of hydrochloric acid to produce 1 mole of sodium chloride.
First, we calculate the number of moles of sodium hydroxide using its molar mass:
Moles of NaOH = (Mass of NaOH ÷ Molar mass of NaOH) = (10 g ÷ 40.00 g/mol)
= 0.25 moles
Since the ratio of moles of sodium hydroxide to moles of sodium chloride is 1:1, the number of moles of sodium chloride produced is:
Moles of NaCl = Moles of NaOH = 0.25 moles
Finally, we convert the moles of sodium chloride to grams using its molar mass:
Mass of NaCl = (Moles of NaCl × Molar mass of NaCl)
= (0.25 moles × 58.44 g/mol) = 14.61 grams
Therefore, the theoretical yield of sodium chloride in the reaction is 14.61 grams.
Frequently asked questions on Sodium hydroxide formula:
- What is sodium hydroxide used for?
Sodium hydroxide has numerous applications across various industries. It is commonly used in the production of soaps, detergents, and cleaning agents. It is also used in water treatment, paper and pulp industry, textile industry, and as a pH regulator in various chemical processes. Sodium hydroxide is also employed in the manufacturing of food products, petroleum refining, and as a drain cleaner.
- Can sodium hydroxide be used for unclogging drains?
Yes, sodium hydroxide can be used as a drain cleaner. It is a strong base that reacts with fats, oils, and other organic matter in the clogged drains, breaking them down and clearing the blockage. However, it should be used carefully and following the instructions provided, as it can cause damage to certain types of pipes or fixtures.
- Can sodium hydroxide be used for skin care?
Sodium hydroxide is used in small quantities in certain skincare products, such as soaps and cleansers. However, it is important to note that sodium hydroxide is a caustic substance, and direct contact with concentrated solutions can be harmful to the skin. In skincare products, sodium hydroxide is used in controlled amounts and is neutralized during the manufacturing process to ensure it is safe for use.
- What is the difference between sodium hydroxide and potassium hydroxide?
Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are both strong bases, but they differ in their chemical compositions. Sodium hydroxide contains sodium (Na+) ions, while potassium hydroxide contains potassium (K+) ions. They have slightly different properties and applications, although they can both be used in similar ways, such as in the production of soaps and cleaning agents.
- Can sodium hydroxide react with acids?
Yes, sodium hydroxide reacts with acids in a neutralization reaction. The hydroxide ions (OH–) from sodium hydroxide combine with the hydrogen ions (H+) from the acid to form water, while the remaining components form a salt. This reaction helps to neutralize the acidic properties of the acid.


