Prepare 250 ml of a N/10 Solution of Oxalic Acid from Crystalline Oxalic Acid
Theory
Apparatus
Watch glass, analytical balance, weight box, fractional weight box, 250 ml beaker, glass rod, 250 ml measuring flask and wash bottle.
Chemical Required
Oxalic acid crystals and distilled water.
Procedure
- Take a watch glass, wash it with distilled water and then dry it.
- Weigh the clean and dried watch glass accurately and record its weight in the note book.
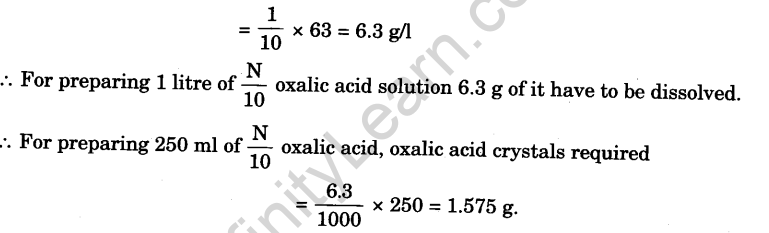
- Weigh 3.150 g oxalic acid on the watch glass accurately and record this weight in the note-book.
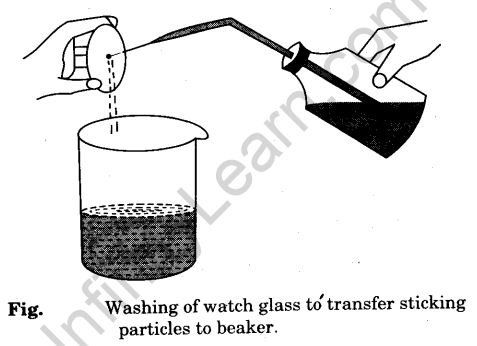
- Transfer gently and carefully the oxalic acid from the watch glass into a clean 250 ml beaker. Wash the watch glass with distilled water with the help of a wash bottle to transfer the particles sticking to it into the beaker [Fig].
The volume of distilled water for this purpose should not be more than 50 ml. - Dissolve oxalic acid crystals in the beaker by gentle stirring with a clean glass rod.
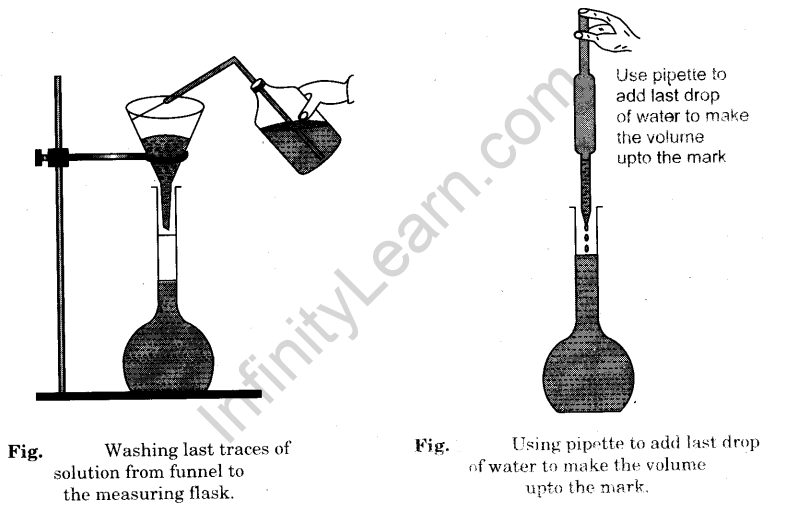
- When the oxalic acid in the beaker is completely dissolved, transfer carefully the entire solution from the beaker into a 250 ml measuring flask (volumetric flask) with the help of a funnel [Fig].
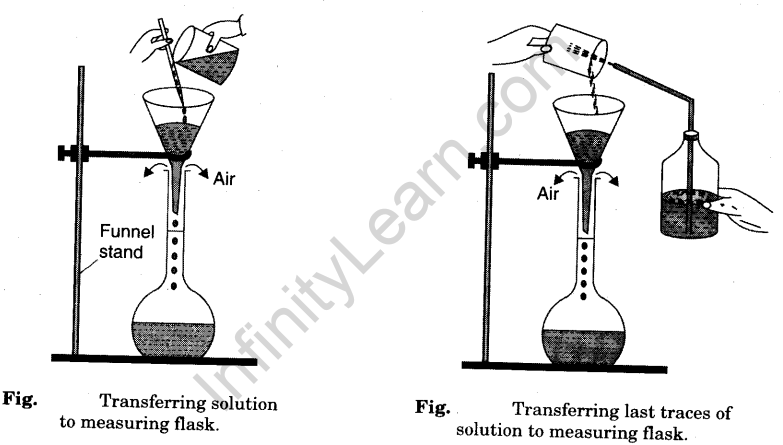
- Wash the beaker with distilled water. Transfer the washings into the measuring flask [Fig].
- Finally wash the funnel well with distilled water with the help of a wash bottle to transfer the solution sticking to the funnel into the measuring flask [Fig].
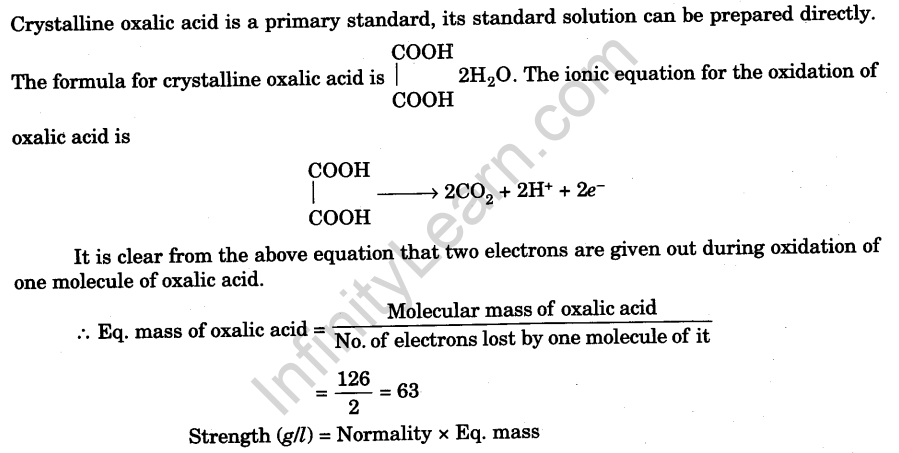
- Add enough distilled water to the measuring flask carefully, up to just below the etched mark on it, with the help of a wash bottle.
- Add the last few drops of distilled water with a pipette until the lower level of the meniscus just touches the mark on the measuring flask [Fig].
- Stopper the measuring flask and shake gently to make the solution uniform through-out. Label it as oxalic acid solution.