Table of Contents
Class 8 Science NCERT Solutions for Chapter 6 Combustion and Flame
NCERT solutions for class 8 Science chapter 6 Combustion and Flame is an essential resource for students studying in class 8. This chapter from the Class 8 Science NCERT textbooks introduces intricate concepts such as ignition, types of combustion, the role of oxygen in combustion, and the characteristics of flames.
Understanding the complexities of combustion and flame properties can be challenging, but these NCERT solutions for class 8 Science chapter 6” are specifically designed to simplify the learning process. These materials assist students in comprehending how various forms of combustion occur, what triggers them, and the substances involved in these processes. Additionally, students gain insight into the concept of ignition temperature and the necessary conditions for combustion to take place.
Safety is another crucial aspect covered in this chapter, focusing on the prevention and management of accidental fires. Through engaging content and detailed explanations, students learn about fire control, the different zones of a flame, and the hazardous pollutants produced during combustion. They also explore the environmental impact of these pollutants, including the phenomenon of acid rain and its detrimental effects on the ecosystem.
These solutions are meticulously crafted to address all the inquiries that may arise in young minds. By practicing the provided questions and engaging with the interactive content, students not only prepare for their exams but also satisfy their curiosity about everyday scientific phenomena.
It is highly encouraged for students to consistently refer to NCERT books for class 8 and these specific solutions to gain a comprehensive understanding of these scientific concepts. The chapter-wise guidance available in NCERT solutions for Class 8 Science, particularly for critical chapters like “Combustion and Flame,” establishes a robust foundation for further scientific exploration. These resources play a pivotal role in helping students appreciate and comprehend the science underlying every flicker of flame.
NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame Free PDF Download
Class 8 Science Chapter 6 Combustion and Flame Questions and Answers
Here are all CBSE class 8 Science Combustion and Flame question answer:
Exercises (Page No. 75)
1. List the conditions under which combustion can take place?
Answer:
For combustion to happen, several conditions must be met:
- Oxygen or air availability: Combustion requires oxygen, which is necessary for the chemical reaction. Without oxygen, the material will not burn. This is why ensuring air supply is crucial for combustion processes.
- Fuel source: For something to burn, there must be fuel. This fuel, which can come in various forms—solid, liquid, or gas—is the material that reacts with oxygen in the combustion process.
- Reaching the ignition temperature: Every fuel has a specific ignition temperature, the minimum temperature at which it begins to burn. This temperature signifies the point at which the material’s heat level is sufficient for it to ignite without any external flame or spark. Ensuring this temperature is reached and maintained is crucial for the combustion process to commence and continue.
2. Fill in the blanks
a) Burning of wood and coal, causes ___________ of air.
b) A liquid fuel, used in homes is ___________
c) Fuel must be heated to its ___________ before it starts burning.
d) Fire produced by oil cannot be controlled by ___________
Answer:
a) Pollution b) Kerosene
c) Ignition temperature d) water
3. Explain how the use of CNG in automobiles has reduced pollution in our cities?
Answer:
Adopting Compressed Natural Gas (CNG) in urban transportation has markedly reduced environmental pollution. CNG, a cleaner alternative to conventional fuels like petrol and diesel, emits fewer harmful gases, including significantly lower levels of carbon monoxide, unburnt hydrocarbons, and particulate matter. These pollutants are major contributors to health issues and urban smog. Also, CNG contains lesser pollutants like sulphur, lead, and benzene, enhancing air quality. An additional benefit is noise reduction, as CNG engines operate more quietly, decreasing noise pollution. Overall, CNG’s use in automobiles is a key strategy in combating urban pollution, contributing significantly to cleaner, more sustainable cities.
4. Compare LPG and wood as fuels?
Answer:
- Energy Efficiency:
- LPG: It offers high energy efficiency, as it produces a large amount of heat per unit, resulting in less fuel consumption.
- Wood: Comparatively inefficient, wood produces less heat per unit, requiring more quantity to generate the same amount of heat as LPG.
- Pollution:
- LPG: Being a cleaner fuel, LPG emits fewer pollutants, significantly reducing the release of carbon monoxide, particulate matter, and other harmful emissions.
- Wood: Burning wood releases more harmful pollutants, including more carbon monoxide and particulate matter, contributing to environmental pollution and health issues.
- Convenience and Safety:
- LPG: Stored in secure cylinders, LPG is easy to transport and store, offering convenience. It provides consistent performance and doesn’t produce residues like ash.
- Wood: Requires significant storage space and isn’t as convenient to transport. It also involves safety risks during transportation and storage, and its burning leaves behind ash residue.
- Sustainability:
- LPG: It is a non-renewable resource; its supply is limited and extraction contributes to environmental degradation.
- Wood: While technically renewable, irresponsible harvesting of wood for fuel can lead to deforestation and ecological imbalance.
- Cost-effectiveness:
- LPG: Although initially more expensive, LPG’s high energy efficiency can make it more cost-effective in the long run.
- Wood: Often cheaper where naturally abundant but less cost-effective in terms of energy output per unit.
In summary, LPG stands out as a more efficient, cleaner, and convenient option compared to wood, despite its higher cost and non-renewability. Wood, while being a traditional fuel and potentially less expensive, poses environmental, health, and logistical challenges that make it less favorable compared to LPG.
5. Give reasons for
a) Water is not used to control fires involving electrical equipment?
b) LPG is a better domestic fuel than wood why?
c) Paper catches fire by it self easily where as a piece of paper wrapped around an aluminum pipe does not?
Answer:
a) Water is not used to control fires involving electrical equipment:
- Water is a good conductor of electricity. Using it to extinguish electrical fires can cause the electricity to spread, potentially leading to electrocution of those in the vicinity.
- Additionally, water can damage electrical components, leading to further hazards or complicating repair and recovery efforts.
b) LPG is a better domestic fuel than wood:
- Efficiency: LPG has a higher energy content per unit volume than wood, meaning it produces more heat using less material, making cooking faster and more efficient.
- Cleanliness: LPG burns more cleanly than wood; it emits fewer pollutants, reducing indoor air pollution, which is beneficial for health.
- Convenience: LPG can be stored in cylinders and used instantly through simple control mechanisms, whereas wood requires storage space, effort for ignition, and tending of the fire.
- Sustainability: Although LPG is a fossil fuel, using it reduces the demand for wood, curbing deforestation and habitat destruction resulting from wood harvesting.
c) Paper catches fire by itself easily, whereas a piece of paper wrapped around an aluminum pipe does not:
- Paper is highly flammable and catches fire easily due to its low ignition temperature. When it’s by itself, heat is rapidly absorbed, and it reaches this ignition point quickly.
- When paper is wrapped around an aluminum pipe, the metal acts as a heat sink, absorbing the heat that the paper would otherwise absorb. This delays the paper from reaching its ignition temperature quickly, making it less likely to catch fire spontaneously. Aluminum’s high thermal conductivity allows it to draw away the heat, preventing the paper from igniting easily.
6. Make a labelled diagram of a candle flame
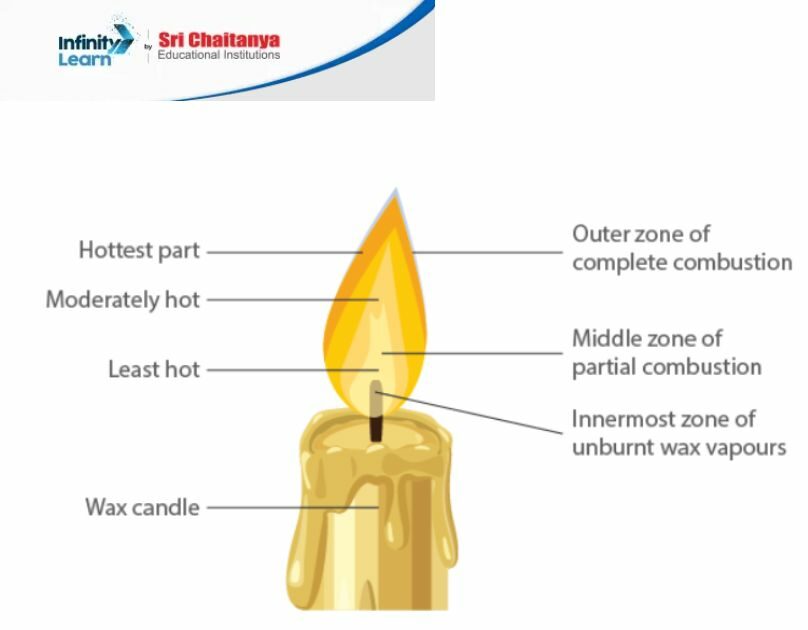
7. Name the unit in which the calorific value of a fuel is expressed?
Answer:
The calorific value of a fuel is typically expressed in a unit called the “Joule” per kilogram (J/kg). In more practical contexts, especially regarding large energy quantities, it might also be expressed in kilojoules per kilogram (kJ/kg) or megajoules per kilogram (MJ/kg). This unit of measurement represents the amount of energy that can be generated when a certain amount of fuel is completely combusted.
8. Explain how CO2is able to control fires?
Answer:
Carbon dioxide (CO2) is effective in extinguishing fires by smothering them. It displaces oxygen from the area surrounding the fire, depriving the fire of the oxygen it needs to sustain combustion. CO2, being heavier than oxygen, covers the fire like a blanket. As the oxygen level drops, the fire diminishes and eventually goes out. Additionally, CO2 does not support combustion and can reduce the heat of the fire as it is released, further helping to eliminate the fire.
9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily explain?
Answer:
Green leaves are full of moisture, which makes them resistant to burning. The water inside the leaves absorbs heat when an attempt is made to ignite the leaves, preventing them from reaching their ignition temperature. This process requires additional energy and consequently, green leaves do not catch fire easily. On the other hand, dry leaves have no moisture content, so they can reach their ignition temperature quickly and easily catch fire.
10. Which zone of a flame does a gold smith use for melting gold and silver and why?
Answer:
A goldsmith uses the outermost zone of a flame, known as the non-luminous or blue zone, to melt gold and silver. This zone is the hottest part of the flame, ensuring the metals reach their melting points effectively. The high temperature in this blue zone is due to complete combustion of the fuel with ample oxygen, ensuring that the energy released is at a maximum, making the process of melting the metals quicker and more efficient.
11. In an experiment 4.5kg of a fuel was completely burnt. The heat produced was measured to be 180,000kj. Calculate the calorific value of the fuel ?
Answer:
Given total mass of fuel = 4.5kg
Total heat produced = 180,000 KJ
Heat produced by burning 1kg of fuel
= 180,000KJ/4.5kg
= 40,000KJ/kg
Calorific value of fuel = 40,000KJ/kg

12. Can the process of rusting be called combustion? discuss.
1. Can the process of rusting be called combustion? Discuss. Rusting, although similar to combustion in that it involves the reaction of a substance (iron) with oxygen, differs significantly in several aspects and is not typically classified as combustion. Here’s why:
- Energy Release: Combustion is an exothermic process that releases energy in the form of heat and sometimes light. Rusting, or the oxidation of iron, is also exothermic but occurs at such a slow rate that the minuscule heat emitted disperses immediately into the surroundings, making it unnoticeable without specialized equipment.
- Reaction Speed: Combustion reactions are generally rapid, producing immediate results, like fire or an explosion. In contrast, rusting is a form of slow oxidation, happening over weeks, months, or years.
- Conditions: Combustion requires a fuel, an oxidant (usually oxygen), and heat (to reach ignition temperature). Rusting requires only iron, oxygen, and water or moisture from the environment.
Due to these distinctions, especially the rate of reaction and the nature of energy release, rusting is not considered combustion
13. Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outer most part of the flame. Whose water will get heated in a shorter time?
In the experiment, Ramesh’s water will get heated in a shorter time compared to Abida’s. Ramesh placed the beaker in the outermost part of the flame, known as the non-luminous zone or the blue zone, which is the hottest part of the flame. This is due to complete combustion occurring in that zone, where the fuel completely reacts with oxygen, producing the maximum amount of heat.
Abida, however, placed her beaker in the yellow part of the flame, known as the luminous zone. This part of the flame is less hot because incomplete combustion occurs here. The presence of unburnt particles in this zone not only reduces the temperature but also causes the yellow color of the flame.
Because Ramesh used the hotter part of the flame, his water would heat up faster than Abida’s.
Also Check: NCERT Solutions for Class 8 Science All Chapters
- Chapter 1 Crop Production and Management
- Chapter 2 Microorganisms: Friend and Foe
- Chapter 3 Synthetic Fibres and Plastics
- Chapter 4 Materials: Metals and Non-Metals
- Chapter 5 Coal and Petroleum
- Chapter 6 Combustion and Flame
- Chapter 7 Conservation of Plants and Animals
- Chapter 8 Cell – Structure and Functions
- Chapter 9 Reproduction in Animals
- Chapter 10 Reaching the Age of Adolescence
- Chapter 11 Force and Pressure
- Chapter 12 Friction
- Chapter 13 Sound
- Chapter 14 Chemical Effects of Electric Current
- Chapter 15 Some Natural Phenomena
- Chapter 16 Light
- Chapter 17 Stars and the Solar System
- Chapter 18 Pollution of Air and Water
Class 8 Science Chapter 6 NCERT Solutions for Combustion and Flame Summary
Combustion and Flame” is an essential chapter that provides a fundamental understanding of how combustion works, the conditions required for it to occur, and its various practical applications. By mastering the concepts presented in this chapter, Class 8 students can not only appreciate the science behind everyday phenomena like burning candles and cooking but also become more aware of fire safety measures and the environmental impact of different fuels. This knowledge forms a crucial foundation for their future scientific exploration and responsible citizenship.
Class 8 Science Chapter 6 NCERT Solutions for Combustion and Flame
NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame gives a clear picture of how to deal with combustion using CNG instead of other fuels. CNG, on the other hand, is a cleaner fuel. CNG can be used in place of diesel, petroleum, or propane/LPG. It has a few of irritating gases in most of the components, in addition to the various energies mentioned previously. The combustion of fossil fuels such as oil produces a large number of unburned carbon particles as well as carbon monoxide, which causes respiratory illnesses.
The following subjects are also covered in this PDF’s solutions. Water is a good power conduit. When water was added to an electrical fire, it just dispersed the electricity even further. An electric jolt could be applied to the person who drenches the flames. LPG is a cleaner fuel than wood because it does not produce smoke or other contaminants. Wood, on the other hand, emits a lot of smoke and exhaust, which pollutes the atmosphere, causes pollution, and causes respiratory problems.
As a result, LPG outperforms wood as a homegrown fuel. Due to its low starting temperature, the paper bursts into flames without the help of anyone else. Because aluminium is a good conductor of power, a piece of paper folded over an aluminium pipe does not burst into flames. At the same time, the paper folded over an aluminium pipe causes the start temperature to rise. In this technique, warmth is transferred from paper to the user.
Also Check: NCERT Solutions for Class 8 All Subjects
- NCERT Solutions for Class 8 Maths
- NCERT Solutions for Class 8 Science
- NCERT Solutions for Class 8 Social Science
- NCERT Solutions for Class 8 English
NCERT Class 8 Science Chapter 6 Solution Weightage Point
In the CBSE examinations, Class 8 Science chapter 6 solution carries a total of 10 marks. The following are the subjects covered in NCERT solutions for Class 8 Science chapter 6:
- Combustion.
- Flame Reduction Techniques
- Combustions come in a variety of shapes and sizes.
- The Function of Oxygen in Combustion
- Temperatures of Ignition.
- Substances that are flammable.
- Controlling the fire.
- The Efficiency of Fuel.
- Flame Definitions and Different Zones
- Pollutants that are hazardous.
- Acid Rain: Its Causes and Consequences
NCERT Solutions for Class 8 Science Chapter 6 Perks Flame and Combustion
NCERT Class 8 Science Chapter 6 has a plethora of advantages. The answers will dispel any uncertainties that candidates may have, and they will also address any future inquiries they may have about these issues. Flame and Combustion and Flame are clearly explained in Class 8 NCERT solutions. These solutions are written in plain language that all pupils may understand. The answers are provided by teachers who are considered experts in this field. Certain are created with the sole purpose of assisting students in achieving the highest possible grade on these topics. The solutions also provide practical examples from Chapter 6 Science Class 8 so that students may tackle any topic in the subject without trouble. For the students’ benefit, all possible topics that may arise in the tests are thoroughly addressed in these solutions.
FAQs on NCERT Class 8 Science Chapter 6 Combustion and Flame
What is NCERT Class 8 Science Chapter 6 about?
This chapter, Combustion and Flame, explores the phenomenon of combustion, types of flames, and the conditions required for combustion.
What is combustion?
Combustion is a chemical reaction in which a substance combines rapidly with oxygen, releasing energy in the form of heat and light.
What are the different types of flames discussed in this chapter?
The chapter discusses three types of flames: a non-luminous flame, a luminous flame, and a candle flame.
What is the role of oxygen in combustion?
Oxygen is crucial for combustion to occur. It supports the chemical reaction between a substance and a fuel, releasing energy in the process.
Why do we see different colors in flames?
Different colors in flames are due to the presence of various metal ions. The energy released during combustion excites these ions, causing them to emit specific colors of light.
What safety measures should one take while dealing with flames and combustion?
Safety measures include using fire extinguishers, maintaining distance from open flames, and avoiding loose clothing while working near fire.
How can I understand the concept of combustion better?
To better understand combustion, try conducting simple experiments involving fire, such as observing different types of flames and their characteristics.
Is this chapter important for exams like Olympiads and competitive exams?
Yes, understanding combustion and flame is fundamental to science and is often included in various competitive exams and Olympiads.
Can I find practical examples of combustion in my daily life?
Yes, combustion is a common process in daily life. Examples include burning fuels for cooking, heating, or driving vehicles.
How can I excel in understanding this chapter?
Reading the chapter thoroughly, taking notes, and practicing related questions will help you grasp the concepts of combustion and flame effectively.









