Sugar, any of numerous sweet, colorless, water-soluble compounds present in the sap of seed plants and the milk of mammals and making up the simplest group of carbohydrates. The most common sugar is sucrose, a crystalline table top and industrial sweetener used in foods and beverages.
As a chemical term, “sugar” usually refers to all carbohydrates of the general formula Cn(H2O)n.
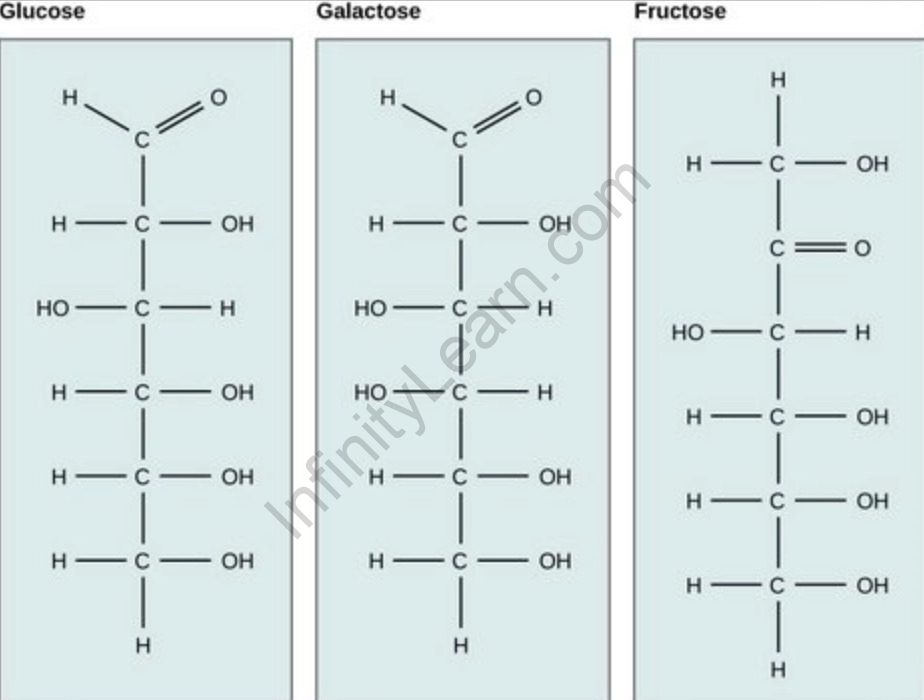
Sucrose is a disaccharide, or double sugar, composed of one molecule of glucose linked to one molecule of fructose. Because one molecule of water (H2O) is lost in the condensation reaction linking glucose to fructose,
For example, Sucrose is represented by the formula C12H22O11 (following the general formula Cn[H2O]n
Physical properties of Sugar:
- Appearance: Sugar is typically found as white crystalline granules or powder. It has a uniform texture and is commonly used as a sweetening agent in various foods and beverages
- Taste: Sugar has a sweet taste and is commonly used to enhance the flavor of foods and drinks. It is a primary source of sweetness in many recipes and is often added to balance or mask other flavors.
- Solubility: Sugar is highly soluble in water, meaning it readily dissolves when mixed with liquid. This property allows sugar to be easily incorporated into various recipes and beverages.
- Melting point: The melting point of sugar is approximately 320-366 degrees Fahrenheit (160-185 degrees Celsius). When heated, sugar undergoes a chemical change and turns into a caramelized liquid.
- Hygroscopicity: Sugar has hygroscopic properties, which means it can absorb moisture from the surrounding environment. This characteristic can affect the texture and shelf life of certain food products.
- Crystallization: Sugar can form different crystal structures based on the conditions under which it is cooled. The size and shape of sugar crystals can impact the texture and mouthfeel of foods, such as confectionery items.
- Sweetness intensity: Sugar is a highly sweet substance, providing a concentrated source of sweetness when added to foods and drinks. The level of sweetness can vary depending on the type of sugar and its concentration in a recipe.
- Density: Sugar has a relatively high density compared to many other common food ingredients. This property can affect its volume and weight when measuring or storing sugar.
- Combustibility: Sugar is combustible and can burn when exposed to an open flame. This property is utilized in various cooking techniques, such as caramelizing sugar to create a caramel sauce or glaze.
- Stability: Sugar is relatively stable under normal storage conditions. However, prolonged exposure to high humidity or moisture can cause clumping or hardening of sugar crystals. Proper storage in airtight containers is recommended to maintain its quality.
Chemical properties of Sugar:
- Chemical Formula: The chemical formula of sugar, specifically table sugar or sucrose, is C12H22O11. It consists of carbon (C), hydrogen (H), and oxygen (O) atoms.
- Molecular Structure: Sugar has a complex molecular structure composed of 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. It forms a cyclic structure in its crystalline form.
- Organic Compound: Sugar is classified as an organic compound because it contains carbon atoms and is derived from living organisms, such as sugar cane or sugar beets.
- Disaccharide: Sugar, specifically sucrose, is a disaccharide composed of two simple sugar molecules, glucose and fructose, bonded together through a glycosidic linkage.
- Carbohydrates: Sugar belongs to the carbohydrate group, which is a major class of biomolecules. Carbohydrates are organic compounds that provide energy to living organisms.
- Sweetness: Sugar exhibits the characteristic sweetness due to its ability to stimulate taste receptors on the tongue. It is one of the primary sources of sweetness in the human diet.
- Fermentable: Sugar can undergo fermentation, a chemical process in which microorganisms such as yeast convert it into alcohol and carbon dioxide. This property is utilized in baking and alcohol production.
- Caramelization: When heated, sugar can undergo caramelization, a chemical reaction in which it decomposes and forms caramel, giving a distinct color, flavor, and aroma. This process occurs at high temperatures and is commonly used in cooking and confectionery.
- Reducing Sugar: Some types of sugars, such as glucose and fructose, are considered reducing sugars because they have the ability to reduce certain compounds, such as Fehling’s solution or Benedict’s reagent, indicating the presence of free aldehyde or ketone functional groups.
- Stability: Sugar is relatively stable under normal conditions. However, when exposed to high temperatures, it can undergo thermal decomposition, leading to the formation of caramel and other byproducts. Additionally, prolonged exposure to acids or enzymes can hydrolyze sugar, breaking it down into its constituent monosaccharides
Solved Examples on Sugar Formula:
Example 1. Calculate the number of oxygen atoms in 5 moles of sucrose (C12H22O11).
Solution: The chemical formula of sucrose, C12H22O11, indicates that there are 11 oxygen atoms in one molecule of sucrose. To calculate the number of oxygen atoms in 5 moles of sucrose, we can use Avogadro’s number (6.022 x 10^23) to convert moles to individual particles:
Number of oxygen atoms = Number of moles x Avogadro’s number x Number of oxygen atoms in one molecule
Number of oxygen atoms = 5 moles x 6.022 x 10^23 molecules/mole x 11 oxygen atoms/molecule
Number of oxygen atoms = 3.3261 x 10^25 atoms
Therefore, there are approximately 3.3261 x 10^25 oxygen atoms in 5 moles of sucrose.
Example 2. Determine the mass of sucrose needed to obtain 1 mole of glucose (C6H12O6) through hydrolysis.
Solution:
In the hydrolysis of sucrose, one mole of sucrose breaks down into one mole of glucose and one mole of fructose. We need to calculate the mass of sucrose that corresponds to one mole of glucose.
The molar mass of glucose (C6H12O6) is calculated as follows:
C: 6 atoms x atomic mass of carbon (12.01 g/mol) = 72.06 g/mol
H: 12 atoms x atomic mass of hydrogen (1.008 g/mol) = 12.096 g/mol
O: 6 atoms x atomic mass of oxygen (16.00 g/mol) = 96.00 g/mol
Now, sum up the masses of each element in glucose:
72.06 g/mol + 12.096 g/mol + 96.00 g/mol = 180.156 g/mol
Since the molar ratio between sucrose and glucose is 1:1, the mass of sucrose needed to obtain 1 mole of glucose is also 180.156 g.
Therefore, to obtain 1 mole of glucose through hydrolysis, you would need 180.156 grams of sucrose.
Frequently asked Questions on Sugar Formula:
1: What is the chemical formula for sugar?
Answer: The chemical formula for sugar depends on the specific type of sugar. However, a common sugar, such as sucrose (table sugar), has the chemical formula C12H22O11.
2: How is sugar classified based on its chemical formula?
Answer: Sugar can be classified into various groups based on its chemical formula. Monosaccharides, such as glucose and fructose, have a simple chemical formula of (CH2O)n, where “n” represents the number of carbon atoms. Disaccharides, like sucrose, are formed by the combination of two monosaccharides through a condensation reaction.
3: Example: Why is the formula for sugar important?
Answer: The formula for sugar is important because it provides information about the composition and structure of the sugar molecule. It helps in understanding the number and types of atoms present, which influences the physical and chemical properties of sugar.
4: Are there different types of sugar with different formulas?
Answer: Yes, there are various types of sugars with different formulas. Some common types include glucose (C6H12O6), fructose (C6H12O6), lactose (C12H22O11), and maltose (C12H22O11). Each sugar type has a unique arrangement of carbon, hydrogen, and oxygen atoms, leading to different properties and applications.
5: Example: How does the formula of sugar impact its sweetness?
Answer: The formula of sugar influences its sweetness to some extent. Different sugar molecules have varying degrees of sweetness. For example, fructose is sweeter than glucose. Additionally, the arrangement and bonding of atoms within the sugar molecule affect how it interacts with taste receptors, contributing to the perception of sweetness.


