Table of Contents
A colorimeter is a tool that detects how different liquids absorb specific colors of light. It measures how much light gets absorbed and how much passes through the liquid. Basically, when you shine a specific color of light through a colored liquid, the colorimeter tells you how much of that light gets absorbed.
This device operates based on Beer-Lambert’s law, which explains how colored substances absorb light.
What Is a Colorimeter?
Colorimetry involves determining the concentration of colored compounds in a liquid solution. A colorimeter is a device sensitive to light, used for measuring how much light passes through or gets absorbed by a sample.
When we measure color, we’re essentially looking at how electromagnetic radiation changes in intensity within the visible light spectrum as it interacts with an object or solution.
This measurement is important because the amount and type of light absorbed or transmitted can tell us about the properties of the solution, such as the concentration of particles within it. This helps in determining the concentration of chemicals.
Colorimeters find applications across various fields like chemistry and biology. They are used for tasks such as measuring solution concentrations, observing reaction rates, monitoring bacterial culture growth, and ensuring laboratory quality control. Now, let’s delve into the components, principles, and applications of this device.
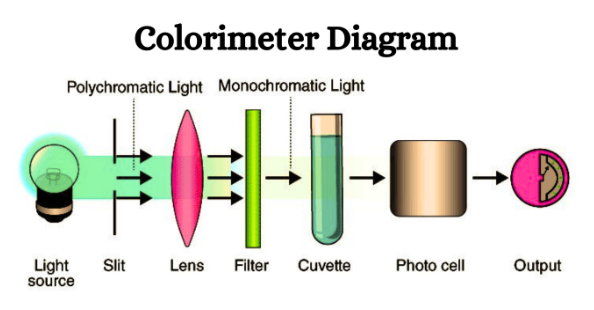
Colorimeter Diagram
The colorimeter consists of several main parts:
Light Source: Typically, colorimeters use a tungsten filament as their light source.
Monochromator: This component separates light into different wavelengths and picks out the specific wavelength being observed.
Sample Holder: The sample holder is where the color sample solution, usually held in cuvettes or test tubes made of glass, is placed for measurement.
Photo Detector System: When light enters this system, it creates an electrical signal. This signal is then displayed as a reading on the galvanometer.
Measuring Device: The galvanometer serves as the measuring device, converting the electrical signals into readings that indicate the light’s intensity.
Colorimeter Principles
Photometry is a method used to measure light. When light beams pass through a liquid solution, three things happen:
- Some of the light bounces back, called Ir.
- Some of the light gets absorbed, known as Ia.
- The remaining light passes through, referred to as It.
Therefore, Io = Ir + Ia + It
To find out how much light is absorbed (Ia), we only need to measure Io and It. We can ignore the reflected light (Ir) because we keep it constant during measurement.
Colorimeters operate based on two fundamental principles of photometry. Let’s break them down:
Beer’s Law:
This law states that the level of light absorbed corresponds directly to the concentration of the solute in the solution. In simpler terms, the more concentrated the solution, the more light it absorbs.
Formula: Log10 Io/It = asc
Where:
‘as’ represents the absorbency index.
‘c’ stands for the concentration of the solution.
Lambert’s Law:
This law explains that the light absorption is linked to both the length and thickness of the solution being analyzed. Essentially, the longer or thicker the solution, the more light it absorbs.
Formula: A = log10 Io/It = asb
Where:
‘A’ denotes the test absorbance.
‘as’ represents the standard absorbance.
‘b’ stands for the length or thickness of the solution.
Working of Colorimeter
To understand how a colorimeter functions, let’s break it down into steps.
Step 1: Calibration
Before starting any experiment, it’s crucial to calibrate the colorimeter. This involves using standard solutions of known concentrations. These solutions are placed in cuvettes and inserted into the colorimeter.
Step 2: Light Path
A specific wavelength of light, tailored to the sample, travels through the solution. Along its path, the light passes through various filters and lenses. These components help isolate the desired wavelength, allowing it to reach the test cuvette.
Step 3: Interaction with Solution
As the light interacts with the solution, it can be transmitted, reflected, or absorbed. The transmitted light is measured by a photodetector system, which converts it into electrical signals. These signals are then sent to a galvanometer.
Step 4: Display
The galvanometer converts the electrical signals into a digital display, showing the intensity of the transmitted light.
Step 5: Calculating Concentration
To determine the concentration of a substance in the test solution, we use the formula:
A = ∈cl
Where “∈” and “l” are constants for the standard and test solutions.
Using the equations:
AT = CT
AS = CS
We derive the colorimeter formula:
AT × CS = AS × CT
CT = (AT/AS) × CT
Where:
AT = Optical density/absorbance of the test solution
AS = Absorbance/optical density of the standard solution
CT = Concentration of the test solution
CS = Standard concentration.
Understanding these steps helps in grasping how a colorimeter operates and how to interpret its results effectively.
Colorimeter Uses
The Colorimeter serves various purposes across different industries:
- Medical Industry: Colorimeters estimate colors in blood, urine, spinal fluid, plasma, and serum for medical analysis.
- Visual Technology: They analyze color and brightness in screens like mobiles, computers, and TVs to enhance user viewing experience.
- Paints and Textiles: Used in these industries for color analysis.
- Food Industry: Employed to assess food and its processing.
- Printing Industry: Measures print paper and ink quality.
- Water Quality Testing: Colorimeters help in checking water quality and detecting substances like chlorine, fluorine, cyanide, iron, and molybdenum.
- Jewelry: Utilized for measuring diamond quality.
- Healthcare: Measures haemoglobin concentration in blood samples.
- Agriculture: Assists in monitoring soil nutrient concentration for better plant growth.
- Pharmaceuticals: Identifies substandard products and drugs.
Applications of Colorimeter
- Colorimeters are widely used for monitoring bacterial growth and yeast culture.
- They provide accurate results for assessing the color of bird plumage.
- Colorimeters measure the color of various foods and beverages, including sugar and vegetable products.
- Advanced colorimeters can also measure colors used in fax machines, copy machines, and printers.
- In chemistry labs, colorimeters are essential for basic research.
- They help test water quality by analyzing chemicals like fluoride, zinc, chlorine, etc.
- Colorimeters aid in determining plant nutrient concentrations such as nitrate, ammonia, and phosphorus in soil.
- They are used to measure hemoglobin levels in blood.
- Colorimetry is crucial in textile manufacturing, color printing, and paint production for quality inspection.
Advantages and Disadvantages of Colorimeter
Advantages:
- A colorimeter is a budget-friendly tool for checking quality effectively.
- You can easily carry portable colorimeters.
- Using a color difference meter gives precise data that can be traced, providing accurate results.
- No need for an expert to operate it.
- Results are available in less than a second.
Disadvantages:
- Identifying colorless substances’ concentration can be time-consuming.
- Colorimeters only work for visible light (400nm to 700nm), not UV or infrared.
- More sample is needed for analysis.
- Preparing a standard solution is necessary.
- Some surfaces reflect light, making measurements tricky.
Precautions Before Using Colorimeter
The colorimeter is a handy tool for measuring colored substances in a solution. To keep it in good shape, handle it carefully and regularly maintain it to prevent dirt or damage.
Here’s how to take care of your colorimeter:
- Always remove the cuvette when not using the instrument.
- If you notice any optical marks on the cuvette, gently clean them with tissue paper.
- Switch off the colorimeter when it’s not in use to extend the lamp’s lifespan and save energy.
- After use, disconnect the plug from the switchboard and cover the colorimeter with its protective cover.
- Check the main power adapter and cable regularly for any signs of wear or tear. Even minor damage can harm the machine and pose risks.
- Replace the instrument if it’s damaged.
- Store it in cool places at room temperature.
- Avoid placing it near harmful chemicals or burning materials.
Difference Between Colorimeter and Spectrophotometer
Colorimeters and spectrophotometers share similarities, but they serve distinct purposes. Spectrophotometers, being powerful tools, excel in providing detailed color data, making them ideal for research and lab work. On the other hand, colorimeters are simpler and commonly used in production and quality control settings.
Here are some key differences:
- Capability and Usage: Spectrophotometers offer comprehensive color measurements, including spectral data, suitable for precise research and lab applications. Colorimeters, simpler in design, find their place in production and quality control tasks.
- Versatility: Spectrophotometers boast adjustable features, making them suitable for various sample types and measurement needs.
- Cost: Spectrophotometers tend to be pricier than colorimeters due to their advanced technology.
- Accuracy: Spectrophotometers are known for their high accuracy and precision, whereas colorimeters may not offer the same level of exactness.
FAQs on Calorimeter
What is the basic principle behind a colorimeter?
A colorimeter detects how different liquids absorb specific colors of light, providing insights into the concentration of colored compounds in a solution.
How does Beer-Lambert's law relate to the operation of a colorimeter?
Beer-Lambert's law explains the relationship between the concentration of a solute in a solution and the amount of light it absorbs, forming the basis for colorimeter measurements.
What are the main components of a colorimeter?
A colorimeter typically consists of a light source, monochromator, sample holder, photo detector system, and measuring device (galvanometer).
What are the primary applications of colorimeters across industries?
Colorimeters are extensively used in fields such as medical analysis, visual technology, paints and textiles, food processing, printing, water quality testing, jewelry assessment, healthcare, agriculture, and pharmaceuticals.
What are the advantages and disadvantages of using a colorimeter?
Advantages include affordability, portability, precise data, ease of operation, and quick results. Disadvantages may include time-consuming analysis for colorless substances, limitations to visible light spectrum, sample quantity requirements, and the need for standard solutions.









