1.RNA is different from DNA because RNA contains (2002)
(1)ribose sugar and thymine
(2)ribose sugar and uracil
(3)deoxyribose sugar and thymine
(4)deoxyribose sugar and uracil
Ans.(2) RNA has ribose sugar and uracil base.
2.The functional group, which is found in amino acid is (2002)
(1)- COOH group 2) – NH2 group
3) – CH3 group 4) both (1) and (2)
Ans.(4) Amino acids contain – NH2 and – COOH groups.
3.Polymer formation from monomers starts by (2002)
(1)condensation reaction between monomers
(2)coordinate reaction between monomers
(3)conversion of monomer to monomer ions by protons •
(4)hydrolysis of monomers
Ans.(1)Polymerisation starts either by condensation or addition reactions between monomers.
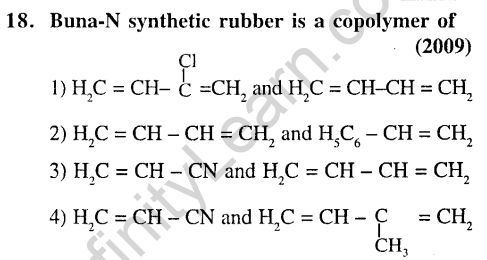
4.Nylon threads are made of (2003)
(1)Polyester polymer 2) Polyamide polymer
(3)Polyethylene polymer
(4)Polyvinyl polymer
Ans.(2)Nylon is a polyamide.
5.Complete hydrolysis of cellulose gives (2003)
(1)D-ribose 2) D-glucose
3) L-glucose 4) D-fructose
Ans.
![]()
6.The reason for double helical structure of DNA is operation of (2003)
(1)dipole – dipole interaction
(2)hydrogen bonding
(3)electrostatic attractions
(4)van der Waal’s forces
Ans.(2) Helix is stabilised by hydrogen bonds.
7.Which base is present in RNA but not in DNA?
1) Uracil 2) Thymine (2004)
3) Guanine 4) Cytosine
Ans.(1)Uracil is not present in DNA.
Ans.
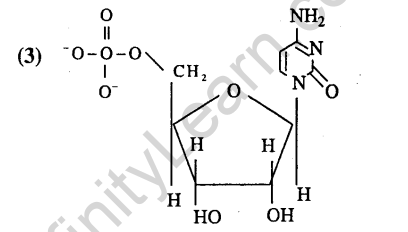
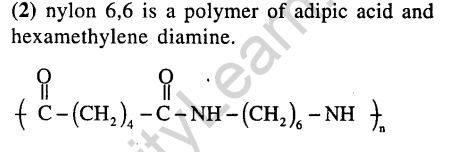
9.Which of the following is a polyamide? (2005)
1) Teflon . 2) Nylon – 6,6
3) Terylene 4) Bakelite
Ans.

10.Which of the following is fully fluorinated polymer ? (2005)
1) Neoprene 2) Teflon
3) Thiokol 4) PVC
Ans.(2) Polytetrafluoroethylene.
11.The term anomers of glucose refers to (2006)
(1)isomers of glucose that differ in configurations at carbons one and four(C-land C-4)
(2)a mixture of (D)-glucose and (L)-glucose
(3)enantiomers of glucose
(4)isomers of glucose that differ in configuration at carbon one (C-l)
Ans.(4) Anomer is specific example of stereoisomer. Carbon atom at position 1 is anomeric.
12.The pyrimidine bases present in DNA are (2006)
(1)cytosine and adenine
(2)cytosine and guanine
(3)cytosine and thymine
(4)cytosine and uracil
Ans.(3) DNA has two pyrimidine bases. Thymine and cytosine.
13.The secondary structure of a protein refers to
(1)a -helical backbone
(2)hydrophobic interactions
(3)sequence of a-amino acids
(4)fixed configuration of the polypeptide backbone
Ans.(1) Secondary structure of proteins involves a-helical back bond and R-sheet structures, These structures are formed as a result of l Hydrogen bonding between different peptide groups in the molecule.
Ans.
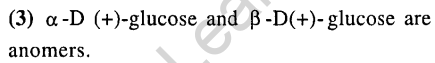
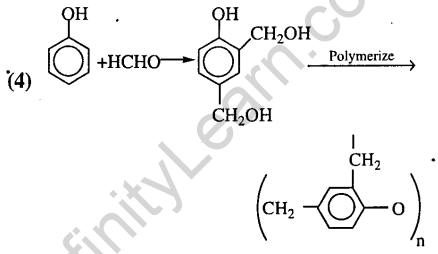
15.Bakelite is obtained from phenol by reacting with (2008)
1) (CH2OH)2 2) CH3CHO
3) CH3COCH3 4) HCHO
Ans.
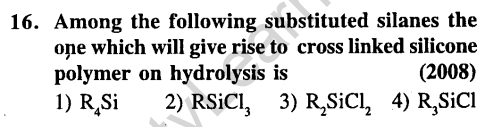
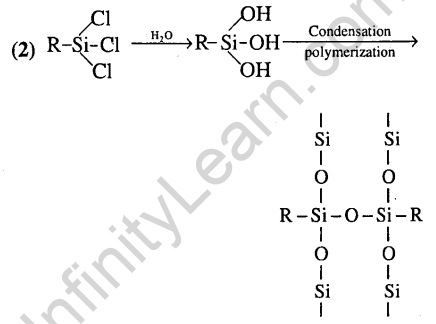
Ans.
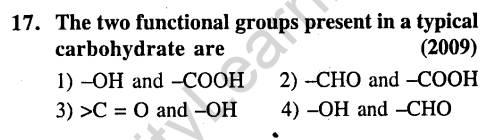
Ans.(3) Carbohydrates are polyhydroxy carbonyl compounds.
Ans.(3) Carbohydrates are polyhydroxy carbonyl compounds.
19.Biuret test is not given by (2010)
1) carbohydrates 2) polypeptides
3) urea 4) proteins
Ans.(1) Biuret is a test characteristic of amide linkage. Urea also has amide linkage like proteins.
20.The polymer containing strong intermolecular forces e.g. hydrogen bonding, is (2010)
1) teflon 2) nylon 6,6
3) polystyrene 4) natural rubber
Ans.
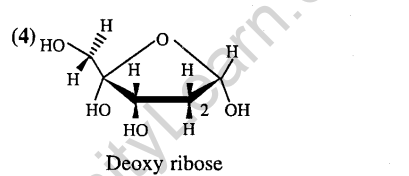
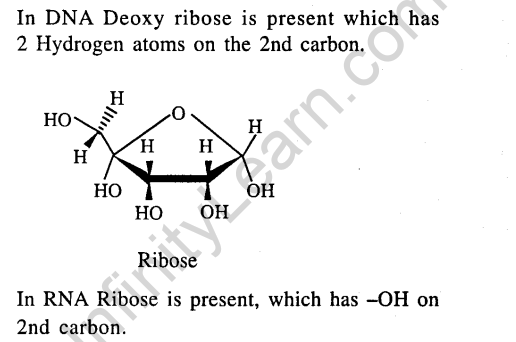
21.The presence or absence of hydroxy group on which carbon atom of sugar differentiates RNA and DNA ? (2011)
1) 3rd 2) 4th 3) 1st 4) 2nd
Ans.
22.Which of the following compounds can be detected by Molish’s test ? (2012)
1)Nitro Compounds 2) Sugars
3) Amines 4) Primary alcohols
Ans.(2) Molish test for carbohydrates : when a drop or two drops of alcoholic solution of oc- naphthol is added to sugar solution and then cone. H2S04 is added along the sides of test tube, formation of violet ring at the junction of two liquids conforms the sugar.

Ans.(3) Lewis acids can initiate the cationic polymerisation.
24.Which one of the following statements is correct ? (2012)
(1)All amino acids except lysine are optically active
(2)All amino acids are optically active
(3)All amino acids except glycine are optically active
(4)All amino acids except glutamic acids are optically active
Ans.(3) Lewis acids can initiate the cationic polymerisation.
25.Synthesis of each molecule of glucose in photosynthesis involves (2013)
1) 18 molecules of ATP 2) 10 molecules of ATP
3) 8 molecules of ATP 4) 6 molecules of ATP
Ans.1) Eighteen ATP units.
26.Which one is classified as a condensation polymer ? (2014)
1) Acrylonitrile 2) Dacron
3) Neoprene 4) Teflon
Ans.(2) Dacron is the condensation polymer Neoprene, teflon and acrylonitrile are addition polymers.
27.Which one of the following bases is not present in DNA ? (2014)
1) Thymine 2) Quinoline
3) Adenine 4) Cytosine
Ans.(2) Quinoline is the base which is not present in DNA.
28.Which polymer is used in the manufacture of paints and lacquers ? (2015)
1) Bakelite 2) Glyptal
3) Polypropene 4) Poly vinyl chloride
Ans.(2) Uses of polymer glyptal.
29.Which of the vitamins given below is water soluble ? (2015)
1) Vitamin C 2) Vitamin D
3) Vitamin E 4) Vitamin K
Ans.(1) Vitamin B and Vitamin C are water soluble.



