Table of Contents
Understanding the difference between “orbit” and “orbital” is crucial for students, particularly those in Class 11, as it lays the groundwork for understanding the fundamental concepts in physics and chemistry.
These terms, often used interchangeably, actually represent distinct concepts. An “orbit” is a term primarily used in astronomy and physics, referring to the path that celestial bodies follow due to gravitational forces.
On the other hand, an “orbital” is a concept in chemistry, describes regions around an atom where electrons are most likely to be found. Grasping the difference between orbit and orbital helps students in developing a clearer understanding of how objects behave both in the vast expanses of space and at the subatomic level.
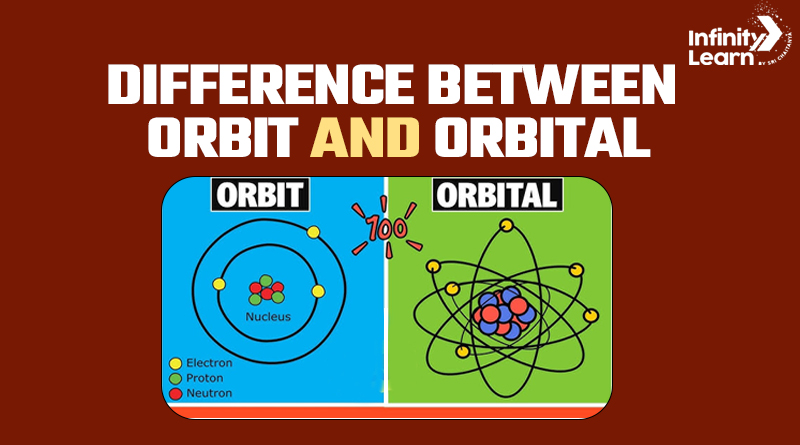
What is Orbit?
An orbit refers to the well-defined path that an object takes around another object due to gravitational forces. It’s a physical path in space, often associated with celestial bodies like planets, moons, or artificial satellites. For instance, the Earth’s orbit around the Sun is an elliptical path that it follows due to the Sun’s gravitational pull.
In the context of Class 11 physics, understanding the difference between orbit and orbital is crucial. When we discuss orbit and orbital differences, we are contrasting a physical path in space (orbit) with a more abstract concept (orbital). Students need to recognize these differences to build a strong foundation in physics and astronomy.
What is Orbital?
The definition of Orbital shifts our focus from the macroscopic orbits of planets to the microscopic world of atoms. An orbital is a region in an atom where there is a high probability of finding electrons. Unlike an orbit, it’s not a defined path but a three-dimensional space with different shapes and orientations.
Understanding the difference between orbit and orbital in Class 11 is essential, especially when exploring the structure of atoms in chemistry. When we talk about orbit and orbital, we are moving from the large-scale movements of celestial bodies to the probabilities and uncertainties in the behavior of subatomic particles.
List the Differences Between Orbit and Orbital
To further clarify, let’s look at a table of difference between orbit and orbital: In this difference between orbit and orbital class 11, students should note how these concepts are applied in different fields and at different scales.
| Orbit | Orbital |
| A physical path in space where a body revolves around another | A region in an atom with a high probability of finding electrons |
| Macroscopic (planets, moons, satellites) | Microscopic (subatomic particles) |
| Defined, precise path | Probabilistic, not a definite path |
| Physics, Astronomy | Chemistry, Quantum Physics |
| Gravitational forces, motion in space | Electron behavior, quantum mechanics |
| An orbit is a fixed path on which electrons revolve around the nucleus. | An orbital is the probable area of finding the maximum density of electrons in an atom. |
| An orbit is a planar representation, i.e., a two dimensional representation. | An orbital is a three dimensional representation. |
| An orbit is non-directional in nature which means the shape of an atom cannot be described by an orbit. | While an orbital can describe the shape of an atom thus is directional in nature. |
| An orbit does not follow the theory of Heisenberg’s Uncertainty Principle. Orbits follow the principles of Bohr-Sommerfeld’s theory. | An orbital follows the theory of Heisenberg’s Uncertainty Principle. |
| An orbit can accommodate 2n2electrons where n represents the number of the orbit or the shell. For example, K shell represents the 1st orbit, L shell represents the 2nd one | An orbital can accommodate the maximum of two electrons only in its sub-levels. The s orbital has only one sub-level, so it can contain only 2 electrons. But the p orbital has 3 sub-levels and thus it can contain upto 6 electrons. |
Orbit – Important Points to Remember
In the context of chemistry, an orbit is defined as the fixed path on which an electron moves or revolves around an atom’s nucleus. It represents a simpler, planar portrayal of an electron’s movement. This concept is grounded in the idea of circular motion, as it describes the path formed due to the electron’s revolution around the nucleus.
However, it’s important to note that orbits fall short in explaining the shape of molecules. This limitation stems from the non-directional nature of orbits. They offer a simplified view that doesn’t account for the complex spatial orientation of molecules.
Additionally, the concept of an orbit contradicts Heisenberg’s Uncertainty Principle, which states that it is impossible to simultaneously know both the exact position and exact momentum of an electron. This principle underscores a fundamental aspect of quantum mechanics, where certainty and fixed paths, as implied by orbits, are not applicable.
Orbitals – Important Points to Remember
In contrast, the concept of orbitals in chemistry is more nuanced and aligns with the principles of quantum mechanics. There are four primary types of orbitals: sharp (s), principal (p), diffuse (d), and fundamental (f). These orbitals are not just varied in type but also exhibit different combinations within each shell of an atom.
For example, within the n=1 shell, only s orbitals are present. In the n=2 shell, both s and p orbitals exist. As we move to the n=3 shell, it encompasses s, p, and d orbitals. For n=4 and higher shells, all four types of orbitals can be found. This organization reflects the increasing complexity and energy levels of electrons as one moves to higher shells.
Orbitals are part of an empirical theory aimed at explaining scientists’ observations regarding bonding and molecular structures. An orbital in chemistry is essentially a wave function. It is a mathematical function that describes the quantum state of a pair of electrons in the vicinity of an atom’s nucleus.
Unlike the fixed path of an orbit, an orbital is depicted as a three-dimensional region where there is a 95% probability of locating an electron. This probabilistic nature of orbitals aligns with the uncertainty and probabilistic predictions of quantum mechanics
The difference between orbit and orbital is significant and understanding these terms helps students in comprehending the fundamental principles of physics and chemistry. Remember, an orbit is about the path of a celestial body, while an orbital relates to the probable location of electrons in an atom.
FAQs on Difference Between Orbit and Orbital
What is the difference between orbit and orbital cavity?
The term 'orbit' in physics refers to the path of an electron around a nucleus or of a celestial body in space. In contrast, 'orbital cavity' relates to human anatomy, specifically the bony socket in the skull that houses the eye and its appendages.
How is orbital different from Bohr's orbit?
An orbital is a quantum mechanical concept describing areas where electrons are likely to be found. Bohr's orbit, from early atomic theory, depicts electrons moving in fixed, circular paths around the nucleus, a concept superseded by the probabilistic nature of orbitals.
What is the difference between orbit, orbital, and node?
An 'orbit' is a defined path of an electron or celestial body. An 'orbital' is a region with a high probability of finding an electron. A 'node,' in this context, is a point in an orbital where the probability of finding an electron is zero.
What is the difference between orbit and orbital?
Orbit and orbital are terms from physics and chemistry. Orbit refers to a defined path of a celestial body or an electron. Orbital, a quantum mechanics concept, describes regions around an atom where electrons are likely found.









