Table of Contents
Definition of Ozone Layer
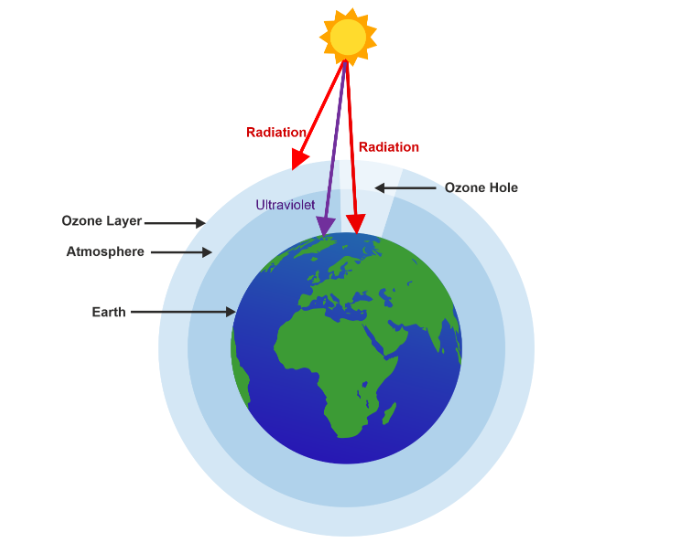
The ozone layer is composed of ozone molecules (O3) that occur naturally in the Earth’s atmosphere. Positioned approximately 10 to 50 kilometers above the Earth’s surface, the ozone layer acts as a filter, absorbing most of the sun’s harmful ultraviolet (UV) radiation. This process shields living organisms from the damaging effects of UV rays, such as skin cancer, cataracts, and immune system suppression.
Ozone depletion
Ozone Layer depletion mechanism is complex and influenced by various factors, including atmospheric dynamics, sunlight intensity, and the presence of other trace gases.
- Emission of Ozone-Depleting Substances (ODS): Human activities release ODS into the atmosphere. The most significant ODS are chlorofluorocarbons (CFCs), halons, carbon tetrachloride, and methyl chloroform. These substances are chemically stable and have long atmospheric lifetimes, allowing them to reach the stratosphere where the ozone layer resides.
- Uplift to the Stratosphere: ODS are typically emitted near the Earth’s surface and then gradually mix and uplift into the stratosphere over several years. This uplift occurs due to atmospheric circulation patterns and natural processes like convection.
- Breakdown by Ultraviolet (UV) Radiation: Once in the stratosphere, ODS are exposed to intense UV radiation from the Sun. The high-energy UV radiation breaks apart the ODS molecules, releasing chlorine (Cl) and bromine (Br) atoms. These atoms are highly reactive and lay a crucial role in ozone destruction.
- Catalytic Destruction of Ozone: Chlorine and bromine atoms are responsible for catalytically destroying ozone molecules. The atoms act as catalysts because they participate in a chain reaction without being consumed themselves. Just one chlorine or bromine atom can destroy thousands of ozone molecules before being removed from the stratosphere, eventually.
- Ozone Destruction Cycle: The destruction cycle begins when a chlorine or bromine atom encounters an ozone molecule (O3). The atom reacts with the ozone molecule, breaking it apart and forming chlorine monoxide (ClO) or bromine monoxide (BrO) and molecular oxygen (O2). The released chlorine or bromine atom then reacts with another ozone molecule, regenerating the chlorine or bromine atom to continue the cycle.
- Depletion of Ozone Layer: As the ozone destruction cycle continues, the concentration of ozone molecules in the stratosphere decreases. This results in a thinning or depletion of the ozone layer, especially in specific regions such as the polar regions during certain times of the year, leading to the formation of the ozone hole.
- Natural Repair Processes: Natural processes also play a role in the ozone depletion mechanism. For instance, nitrogen oxides (NOx) and hydroxyl (OH) radicals help remove chlorine and bromine atoms from the stratosphere. Additionally, the polar vortex, a persistent wind pattern, contributes to the isolation and cooling of air masses over polar regions, facilitating the conditions for ozone depletion.
Causes of Ozone Layer Depletion
- Chlorofluorocarbons (CFCs) and Halons: One of the primary causes of ozone depletion is the release of human-made chemicals, such as CFCs and halons. These substances were widely used in various applications, including aerosol propellants, refrigerants, and fire extinguishers. Once released into the atmosphere, CFCs and halons can rise to the stratosphere and interact with ozone molecules, breaking them down and depleting the ozone layer.
- Methyl Chloroform and Carbon Tetrachloride: Industrial solvents, such as methyl chloroform and carbon tetrachloride, also contribute to ozone depletion. Although their ozone depletion potentials are lower compared to CFCs, their cumulative effect cannot be disregarded.
Ozone hole
The ozone hole refers to a severe depletion of the ozone layer over certain regions, particularly the polar regions, during specific times of the year. It is characterized by a significant drop in ozone concentration, leading to a “hole” or a region with extremely low levels of ozone. The ozone hole primarily occurs over Antarctica during the Southern Hemisphere’s spring (September to November). It is most pronounced during September when the polar vortex, a persistent wind pattern, traps cold air over the continent and isolates it from warmer air masses. However, smaller-scale ozone holes have also been observed over the Arctic region.
Consequences of Ozone Layer Depletion
- Increased UV Radiation: The thinning of the ozone layer allows more UV radiation to reach the Earth’s surface. Prolonged exposure to high levels of UV radiation can lead to sunburns, skin cancer, cataracts, and weakened immune systems in humans. It also affects marine life, terrestrial plants, and phytoplankton, disrupting ecosystems and leading to reduced crop yields.
- Climate Change: The interaction between ozone depletion and climate change is complex. While ozone depletion itself does not directly cause global warming, certain ozone-depleting substances, such as CFCs, are also potent greenhouse gases. The phasing out of these substances contributes to mitigating climate change, aligning efforts to address both environmental challenges.
Control measures of Ozone Layer Depletion
- Montreal Protocol: In response to the growing concern over ozone depletion, the international community established the Montreal Protocol on Substances that Deplete the Ozone Layer in 1987. The protocol aimed to phase out the production and consumption of ozone-depleting substances and has been highly successful. The protocol’s implementation has led to a significant reduction in the production and use of such substances globally. Ozone hole is not a permanent phenomenon. It exhibits a seasonal pattern and varies in intensity from year to year. Thanks to international efforts under the Montreal Protocol, which phased out ozone-depleting substances, the size of the ozone hole has been decreasing gradually, and signs of recovery are observed.
- Alternative Technologies: As part of the Montreal Protocol, efforts have focused on finding environmentally friendly alternatives to ozone-depleting substances. Innovations in refrigeration, air conditioning, aerosol propellants, and fire suppression systems have led to the development of safer and more sustainable substitutes.
- Public Awareness and Education: Raising awareness about the ozone layer depletion and its consequences is crucial. Governments, non-governmental organizations, and educational institutions play a vital role in educating the public about the importance of ozone protection and encouraging environmentally responsible practices.
Summary
The ozone layer is a layer in the Earth’s atmosphere composed of ozone molecules that absorb harmful ultraviolet (UV) radiation from the sun. It acts as a filter, protecting living organisms from the damaging effects of UV rays. Ozone depletion is a complex process influenced by factors such as human activities, emission of ozone-depleting substances (ODS) like chlorofluorocarbons (CFCs) and halons, uplift of ODS to the stratosphere, breakdown by UV radiation, and catalytic destruction of ozone by chlorine and bromine atoms. This depletion leads to a thinning or depletion of the ozone layer, including the formation of the ozone hole over polar regions. The causes of ozone layer depletion include the release of CFCs, halons, methyl chloroform, and carbon tetrachloride into the atmosphere. The consequences of ozone layer depletion include increased UV radiation reaching the Earth’s surface, which can cause sunburns, skin cancer, cataracts, and harm to ecosystems. Ozone depletion is also connected to climate change, and efforts to address it involve the Montreal Protocol, which aims to phase out ozone-depleting substances. Alternative technologies and public awareness and education are also important in mitigating ozone layer depletion. The size of the ozone hole has been decreasing gradually due to international efforts, and signs of recovery are observed.
FAQs on Ozone depletion
What is ozone depletion?
Ozone depletion refers to the gradual thinning of the ozone layer, primarily in the Earth's stratosphere. It occurs when ozone-depleting substances, such as chlorofluorocarbons (CFCs), halons, and certain industrial solvents, are released into the atmosphere and react with ozone molecules, causing them to break down.
What are the main causes of ozone depletion?
The main causes of ozone depletion are human-made chemicals, particularly CFCs and halons. These substances were widely used in industries and consumer products such as aerosol propellants, refrigerants, and fire extinguishers. When released, they rise to the stratosphere and catalytically destroy ozone molecules.
What are the effects of ozone depletion on human health?
Ozone depletion leads to an increase in ultraviolet (UV) radiation reaching the Earth's surface. Overexposure to UV radiation can cause sunburns, skin cancer, cataracts, and weakened immune systems in humans. It is crucial to protect ourselves from excessive UV radiation by using sunscreen, wearing protective clothing, and seeking shade when the sun is strongest.
How does ozone depletion impact the environment?
Ozone depletion has significant consequences for the environment. Increased UV radiation can harm phytoplankton, marine life, and terrestrial plants, leading to reduced productivity and disrupted ecosystems. It can also have adverse effects on agricultural crops and decrease biodiversity in various ecosystems.
What is the Montreal Protocol, and how has it addressed ozone depletion?
The Montreal Protocol on Substances that Deplete the Ozone Layer, established in 1987, is an international treaty aimed at phasing out the production and consumption of ozone-depleting substances. It has been highly successful in reducing the emissions of such substances globally and has led to the gradual recovery of the ozone layer.








