Table of Contents
CBSE Previous Year Question Papers Class 12 Chemistry 2012 Outside Delhi
Time allowed: 3 hours
Maximum Marks: 70
CBSE Previous Year Question Papers Class 12 Chemistry 2012 Outside Delhi Set I
Question 1.
How may the conductivity of an intrinsic semiconductor be increased? [1]
Question 2.
Define ‘peptization’. [1]
Answer:
The process of conversion of freshly prepared precipitate into a colloidal solution by adding suitable electrolyte is called peptization.
Question 3.
How is copper extracted from a low-grade ore of it? [1]
Answer:
Copper from its low-grade ore is leached out using acid or bacteria and then Cu2+ ions in the solution are reduced to copper by treating it with Hydrogen or Iron. This method is called the hydrometallurgy.
Question 4.
Which is a stronger reducing agent, SbH3 or BiH3, and why? [1]
Question 5.
What happens when bromine attacks CH2=CH-CH2-C=CH? [1]
Answer:
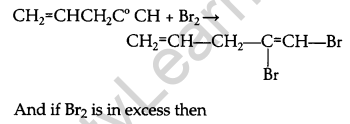
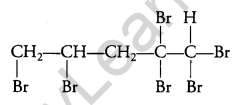
Question 6.
Write the IUPAC name of the following: [1]
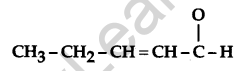
Answer:
Pent-2-enal
Question 7.
Write the structure of the product obtained when glucose is oxidised with nitric acid. [1]
Answer:
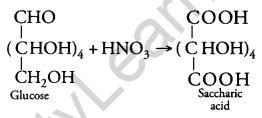
Question 8.
Differentiate between disinfectants and antiseptics. [1]
Answer:
Antiseptics are used on living tissues to kill or prevent the growth of microorganisms. It does not cause any harm to the living tissues e.g. 0.2% solution of phenol.
Disinfectants are used on non-living objects to kill the micro-organisms. They are harmful to the living tissues and hence, cannot be applied to the skin e.g. 1% solution of phenol.
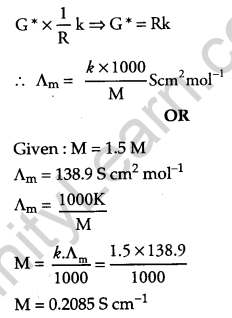
M = 0.2085 S cm-1
Question 9.
Express the relation among cell constant, the resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity? [2]
OR
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2 mol-1. Calculate the conductivity of this solution.
Answer:
CG* = k
Where, C = conductance, G* = cell constant, k = conductivity
Question 10.
A reaction is of second order with respect to a reactant. How is its rate affected if the concentration of the reactant is
(i) doubled
(ii) reduced to half? [2]
Answer:
(i) Rate increases by four times,
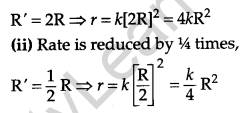
Question 11.
Which methods are usually employed for purifying the following metals: [2]
(i) Nickel
(ii) Germanium.
Mention the principle behind each one of them.
Answer:
(i) Mond’s Process: In this method Nickel form volatile complex with Co which decomposes on heating to give a pure nickel.
(ii) Zone Refining: It is based on the principle that the impurities are more soluble in the molten state than in the solid-state of the metal.
Question 12.
Explain the following facts giving the appropriate reason in each case: [2]
- NF3 is an exothermic compound whereas NCl3 is not.
- All the bond in SF4 is not equivalent.
Answer:
- The bond energy of the F-F bond is lower that of N-F bond So NF3 is an exothermic compound whereas bond energy of Cl-Cl bond is higher than N-Cl bond so NCl3 is an endothermic compound.
- SF4 has a see-saw structure with bond pairs at two equatorial and two axial positions. Hence, all the bonds in SF4 are not equivalent.
Question 13.
Complete the following chemical reaction equations: [2]
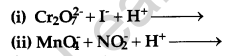
Answer:
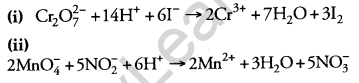
Question 14.
Explain the mechanism of acid catalysed the hydration of an alkene to form corresponding alcohol. [2]
Answer:
Step 1: Carbocation is formed due to the electrophilic attack by H3O+.
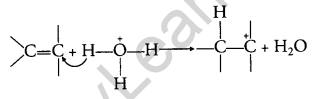
Step 2: Nucleophilic water attacks the carbocation.
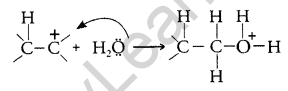
Step 3: Deprotonation to form an alcohol
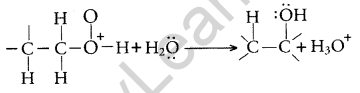
Question 15.
Explain the following behaviours: [2]
- Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
- Ortho-nitrophenol is more acidic than ortho methoxyphenyl.
Answer:
- Due to the formation of hydrogen bonds by alcohols with water molecules.
- The phenoxide ion. is more stable for o-nitrophenol as the nitro group is electron-withdrawing due to resonance while the methoxy group is electron-donating via resonance.
Question 16.
Describe the following giving the relevant chemical equation in each case: [2]
(i) Carbylamine reaction
(ii) Hofmann’s bromamide reaction.
Answer:
(i) Carbylamine Reaction: When aliphatic and aromatic primary amines are heated with chloroform and ethanolic KOH solution, they form isocyanides or carbylamines which are foul-smelling substances.
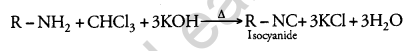
(ii) Hofmann’s Bromamide Reaction: In this reaction, primary amines are prepared by treating an amide with Br2 in an aqueous or alcoholic solution of NaOH
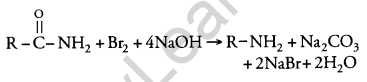
Question 17.
Complete the following reaction equations: [2]
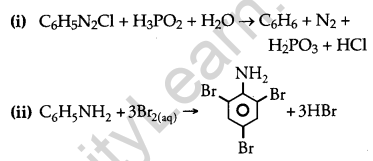
(i) C6H5N2Cl + H3PO2 + H2O →
(ii) C6H5NH2 + Br2(aq.) →
Answer:
Question 18.
What are food preservatives? Name two such substances. [2]
Answer:
Chemicals used to prevent spoilage of food by preventing the growth of microorganisms like bacteria, fungus etc are called food preservatives. Sodium benzoate, nitrogen gas are two such substances which are used as food preservatives.
Question 19.
Copper crystallizes with face centred cubic unit cell. If the radius of the copper atom is 127.8 pm, Calculate the density of the copper metal. [3]
(Atomic mass of Cu = 63.55 u and Avogadro’s number NA = 6.02 × 1023 mol-1)
OR
Iron has a body-centred cubic unit cell with the cell dimension of 286.65 pm. The density of iron is 7.87 g cm-3. Use this information to calculate Avogadro’s number. (Atomic mass of Fe = 56.0 u)
Question 20.
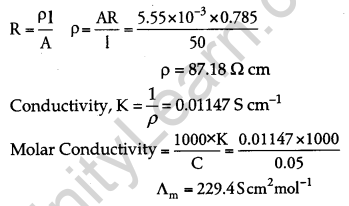
The electrical resistance of a column of 0.05 M NaOH solution of diameter 1 cm and length 50 cm is 5.55 × 103 ohm. Calculate its resistivity, conductivity and molar conductivity. [3]
Answer:
A = πr2 = 3.14 × (0.5)2 = 0.785 cm2, l = 50 cm
Question 21.
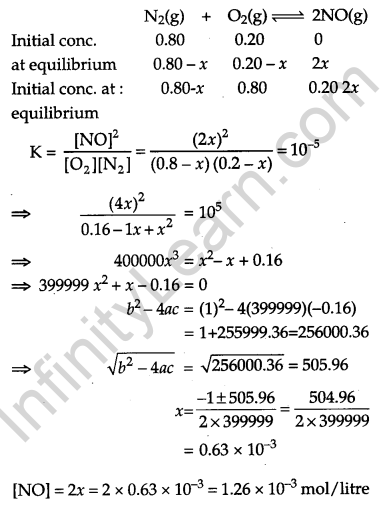
The reaction N2(g) + O2(g) ⇔ 2NO(g) contributes to air pollution whenever a fuel is burnt in the air at a high temperature. At 1500 K, equilibrium constant K for it is 1.0 × 10-5. Suppose in a case [N2] = 0.80 mol L-1 and [O2] = 0.20 mol L-1 before any reaction occurs. Calculate the equilibrium concentrations of the reactants and the product after the mixture has been heated to 1500 K. [3]
Answer:
Question 22.
Explain the following terms giving a suitable example for each: [3]
- Aerosol
- Emulsion
- Micelle.
Answer:
- An aerosol is a colloid in which dispersed phase is a solid and dispersion medium is a gas eg., Dust, smoke.
- An emulsion is a colloid solution in which both the dispersed phase and dispersion medium are in the liquid state, eg., Milk and cod liver oil.
- They are associated colloids showing colloidal behaviour at high concentration and strong electrolytes at low concentration, e.g., Soap.
Question 23.
How would you account for the following: [3]
- Among lanthanoids, Ln (III) compounds are predominant. However, occasionally in solution or in solid compounds, +2 and +4 ions are also obtained.
- The E0(m2+/m) for copper is positive (0.34 V). Copper is the only metal in the first series of transition elements showing this behaviour.
- The metallic radii of the third (5d) series of transition metals are nearly the same as those of the corresponding members of the second series.
Answer:
- Some lanthanoid show +2 and +4 oxidation states in ionic solutions or solid components due to the extra stability that arises because of empty half-filled or fully filled 4f-subshell.
- This is because copper has a high enthalpy of atomization and low enthalpy of hydration. Hence, E0(Cu2+/Cu) is positive.
- This is because of lanthanoid contraction the metallic radii of the third (5d) series of transition metals are nearly the same as those of the corresponding members of the second series.
Question 24.
Name the following coordination entities and draw the structures of their steroisomers: [3]
(i) [Co(en)2Cl2]+ (en = ethan-1,2-diamine)
(ii) [Cr(C2O4)3]3-
(iii) [CO(NH3)3Cl3]
(Atomic numbers Cr = 24, Co = 27)
Answer:
(i) [Co(en)2Cl2]+ dichloridobis (ethan-1,2 diamine) cobalt (III) ion
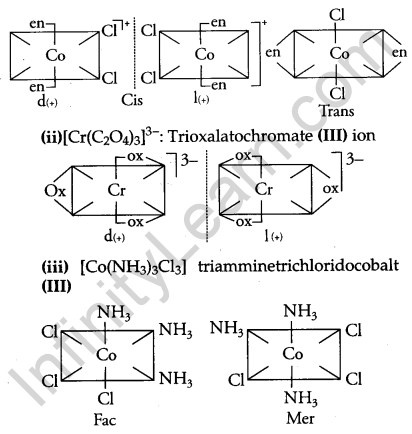
Question 25.
Answer the following questions: [3]
(i) What is meant by the chirality of a compound? Give an example.
(ii) Which one of the following compounds are more easily hydrolyzed by KOH and why?
CH3CHClCH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes SN2 substitution reaction faster and why?
![]()
Answer:
(i) Chirality is the property of a molecule to have non-superimposable mirror images. These molecules contain one asymmetric carbon atom, e.g., Butan-2-ol
(ii) CH3CHClCH2CH3 is more easily hydrolyzed due to the formation of a more stable secondary carbocation.
(iii) CH3CH2CH2Cl undergoes SN2 substitution reaction faster because it is a better leaving group due to its large size and less electronegativity.
Question 26.
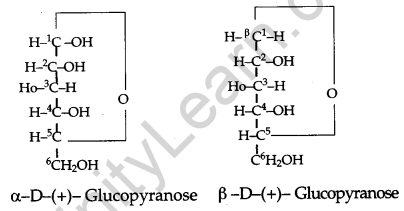
What is essentially the difference between α-glucose and β-glucose? What is meant by pyranose structure of glucose? [3]
Answer:
α-glucose and β-glucose are two cyclic hemiacetal forms of glucose which differ only in the configuration of the hydroxyl group (-OH) at anomeric carbon. Such isomers are called anomers. The six-membered cyclic structure of glucose is called the pyranose structure.
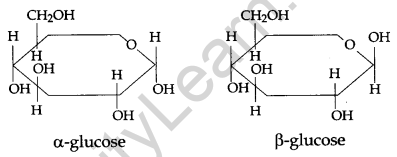
Pyranose Structure of Glucose: The six-membered ring contains oxygen atom because of its resemblance with pyran it is called pyranose form.
Question 27.
Differentiate between thermoplastic and thermosetting polymers. Give one example of each. [3]
Answer:
Thermoplastic Polymers: These polymers do not have cross-links between their chains and hence can be reshaped upon heating eg. Polyethylene, Polypropene etc.
Thermosetting Polymers: These polymers have cross-links between their chains and hence cannot be reshaped upon heating, eg. Bakelite, Melamine etc.
Question 28.
(a) Define the following terms: [5]
(i) Mole fraction
(ii) Ideal solution.
(b) 15.0 g of an unknown molecular material was dissolved in 450 g of water. The resulting solution was found to freeze at -0.34 °C. What is the molar mass of this material? (Kf for water = 1.86 K kg mol-1)
OR
(a) Explain the following:
(i) Henry’s law about the dissolution of a gas in a liquid.
(ii) Boiling point elevation constant for a solvent.
(b) A solution of glycerol (C3H8O3) in water was prepared by dissolving some glycerol in 500 g of water. This solution has a boiling point of 100.42°C. What mass of glycerol was dissolved to make this solution? (Kb for water = 0.512 K kg mol-1).
Answer:
(a) (i) The ratio of number of moles of a solute (components of a mixture) to the total number of moles in the mixture is called a mole
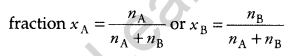
(ii) A solution that obeys Raoult’s law at all temperature and concentration is called an ideal solution.
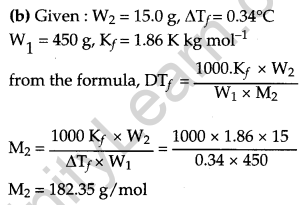
OR
(a) (i) Henry’s law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
i.e., p = KHX
(ii) The boiling point elevation constant for a solvent is defined as the elevation in boiling point when the molality of the solution is unity.
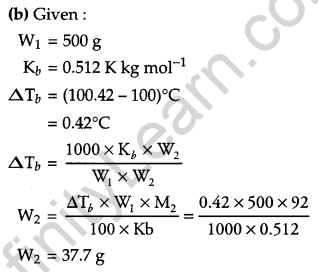
Question 29.
(a) Draw the molecular structures of the following compounds: [2, 3]
(i) N2O5
(ii) XeOF4.
(b) Explain the following observations:
(i) Sulphur has a greater tendency for catenation than oxygen.
(ii) I-Cl is more reactive than I2.
(iii) Despite the lower value of its electron gain enthalpy with a negative sign, fluorine (F2) is a stronger oxidizing agent than Cl2.
OR
(a) Complete the following chemical equations:
(i) Cu + HNO3 (dilute) →
(ii) XeF4 + O2F2 →
(b) Explain the following observations:
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) Oxygen is gas but sulphur a solid.
(iii) The halogens are coloured. Why?
Answer:
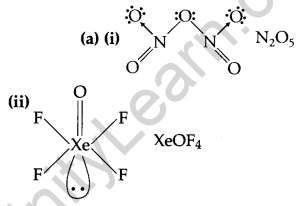
(b) (i) Due to strong S—S bond and less interelectronic repulsion, sulphur has a greater tendency for catenation.
(ii) I—Cl bond is polar and hence more reactive compound to I2 in which I—I bond is non-polar.
(iii) Due to high electronegativity and small size of fluorine, it acts as a stronger oxidizing agent.
OR
(a) (i) 3Cu + 8HNO3 (dil) → 3Cu (NO3)2 + 2NO + 4H2O
(ii) XeF4 + O2F2 → XeF6 + O2
(b) (i) Catenation (i.e. linking of atoms of the same kind with one another) is related to the atom-atom bond energy. Greater the atom-atom bond energy, greater is the catenation. Because of low N—N bond energy (163.8 KJ mol-1) nitrogen shows little tendency for catenation. P—P bond energy (201 × 10-6 KJ/mol) is quite high, hence, it shows more tendency for catenation than nitrogen.
(ii) Oxygen forms pπ – pπ multiple bonds. Due to the small size and high electronegativity oxygen exists as diatomic (O2) molecule. These molecules are held together by weak van der Waals’ forces. Place O2 is a gas at room temperature.
Sulphur because of its bigger size and lower electronegativity, prefer to form S—S single bonds. Further because of stronger S—S than O—O single bonds, sulphur has a much greater tendency for catenation than oxygen. Thus, sulphur because of its higher tendency for catenation and a lower tendency for pπ-πn multiple bonds, forms octa atomic (S8) molecules, having an eight-membered puckered ring structure. Because of bigger size, the force of attraction holding the Ss molecule together are much stronger. Hence, sulphur is a solid at room temperature.
(iii) All halogens are coloured. It is due to the reason that their molecules absorb light in the visible region as a result of which their electrons get excited to higher energy levels while the remaining light is transmitted. The colour of the halogens is actually the colour of this transmitted light.
Question 30.
(a) Write a suitable chemical equation to complete each of the following transformations: [2, 3]
(i) Butan-1-ol to butanoic acid
(ii) 4-Methylacetophenone to benzene-1, 4-dicarboxylic acid
(b) An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro’s reaction. On vigorous oxidation, it gives 1, 2-benzene dicarboxylic acid. Identify the compound.
OR
(a) Give chemical tests to distinguish between:
(i) Propanol and propanone
(ii) Benzaldehyde and acetophenone
(b) Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) Benzoic acid, 3, 4-dinitrobenzoic acid, 4-Methoxy-benzoic acid (acid strength)
(iii) CH3CH3CH(Br)COOH, CH3CH(Br) CH2COOH, (CH3)2CHCOOH(acid strength)
Answer:
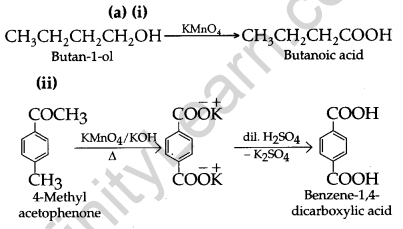
(b) 1. The given compound with molecular formula C9H10O forms a 2, 4-DNP derivative and reduces Tollen’s reagent It must be an aldehyde.
2. As the compound undergoes Cannizzaro reaction, therefore CHO group is directly attached to the benzene ring.
3. On vigorous oxidation, it gives 1, 2-benzene dicarboxylic acid, therefore, it must be an ortho-substituted benzaldehyde.
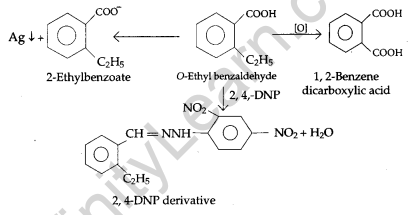
OR
(a) (i) Iodoform Test: This test is given by propanone and not by propanol. Propanone on reacting with hot NaOH/I2 gives a yellow precipitate of CHI3 while propanol does not.
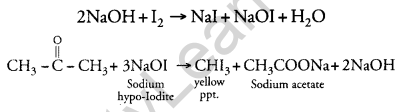
(ii) Silver Mirror Test: Benzaldehyde being an aldehyde reduces Tollens’ reagent to give a silver mirror test but acetophenone being a ketone does not give this test.
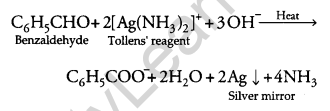
(b) (i) Methyl tert – butyl ketone < Acetone < Acetaldehyde.
(ii) 4-methoxy benzoic acid < benzoic acid < 3, 4 dinitrobenzoic acid
(iii) (CH3)2CHCOOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br)COOH
CBSE Previous Year Question Papers Class 12 Chemistry 2012 Outside Delhi Set II
Note: Except for the following questions, all the remaining questions have been asked in the previous set.
Question 1.
Which stoichiometric defect increases the density of a solid? [1]
Question 2.
What is meant by ‘shape-selective catalysis’? [1]
Answer:
Zeolites are shaped selective catalysts and shape-selective catalysis depends upon the structure of the pores present in the catalyst and size of the reactant and product molecules. ZSM-5 is an example of a shape-selective catalyst, which is used in converting alcohol directly into gasoline.
Question 3.
What is the role of collectors in Froth Floatation process? [1]
Answer:
Collectors help in attachment of ore particle to an air bubble in froth e.g., Sodium xanthates.
Question 6.
Write the IUPAC name of Ph – CH = CH – CHO. [1]
Answer:
3-phenyl prop-2-enal
Question 17.
Explain the cleaning action of soap. Why do soaps not work in hard water? [2]
Answer:
When soap is rubbed on dirty cloth in water, the concentration of soap becomes greater than (CMC) critical micelle concentration, micelle formation takes place. These micelles get adsorb at the dirt or grease
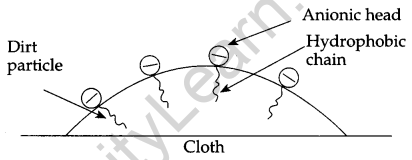
In such a manner that hydrophobic end get adsorbs and anionic head remains at the surface.
Therefore dirt particle becomes negatively charged and when rinsing is done, this particle moves along with water and clothes become free from dirt or grease.
In soap sodium or potassium salts of fatty acids are present e.g., C17H35COO–Na+ (sodium stearate) when it is added in hard water, presence of calcium or magnesium salts makes insoluble salts of Ca or Mg with carboxylate ion called scum which sticks to the cloth as gummy mass.
Question 20.
A voltaic cell is set up at 25°C with the following half cells:
Al/Al3+ (0.001 M) and Ni/Ni2+ (0.50 M)
Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential. [3]
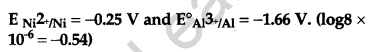
Answer:
Question 23.
Explain the following observations:
- Many of the transition elements are known to form interstitial compounds.
- There is a general increase in density from titanium (Z = 22) to copper (Z = 29).
- The members of the actinoid series exhibit a larger number of oxidation states than the corresponding members of the lanthanoid series. [3]
Answer:
- Transition elements are known to form interstitial compounds because these elements are capable of entrapping smaller atoms of other elements such as H, C and N in the interstitial sites in their crystal lattice.
- It is due to an increase in atomic mass whereas atomic size decreases from Ti to Cu. Therefore, density goes on increasing.
- Because of the comparable energies of 5f, 6d and 7s orbitals in actinoids, they exhibit a larger number of oxidation states than the corresponding members of lanthanoid series.
Question 27.
Explain the following terms giving a suitable example for each: [3]
- Elastomers
- Condensation polymers
- Addition polymers.
Answer:
- Elastomers: Their polymer chain is held together by weakest intermolecular forces so that they can be stretched. That is why they exhibit elastic properties and are rubber-like solids, eg., Buna-S, Buna-N etc.
- Condensation Polymers: They are formed by repeated condensation reaction between two different bi or tri-functional monomeric units with the elimination of smaller molecules such as water, alcohol, HCl etc. e.g.,: Nylon 6, 6.
- Addition Polymers: They are formed by the repeated addition of monomer molecules having double or triple bonds, eg: Polyethylene.
Question 30.
(a) Draw the structures of the following molecules: [5]
(i) H3PCV
(ii) ClF3.
(b) Explain the following observations:
(i) Nitrogen is much less reactive than phosphorus.
(ii) Despite having greater polarity, hydrogen fluoride boils at a lower temperature than water.
(iii) Sulphur has a greater tendency for catenation than oxygen in the same group.
OR
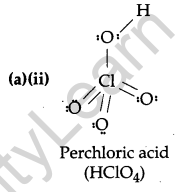
(a) Draw the structures of the following molecules:
(i) N2O5
(ii) HClO4.
(b) Explain the following observations:
(i) H2S is more acidic than H2O.
(ii) Fluorine does not exhibit any positive oxidation state.
(iii) Helium forms no real chemical compound.
Answer:
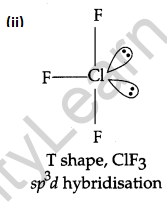
(b) (i) Due to N=N triple bond in N2 molecule, it is inert and hence is less reactive as compared to phosphorous which has P-P single bond, which makes it more reactive.
(ii) In the case of HF, an average of two intermolecular hydrogen bonds are present. As a result, Vander Waals forces of attraction increases in the water molecule and hence, boiling point increases.
(iii) Because S-S bond is stronger than 0-0 bonds as there is more interelectronic repulsion in O-O than in S-S.
OR
(b) (i) Because bond dissociation enthalpy of H-S bond in H2S is less than that of H-O bond in H2O.
(ii) Fluorine is the most electronegative element in nature and it has no d-orbitals and therefore, there is no scope for electron promotion.
Hence, it can show only -1 oxidation state in its compounds.
(iii) Because it is an inert gas and has very high ionization enthalpy, therefore no real chemical compound of helium is known.
CBSE Previous Year Question Papers Class 12 Chemistry 2012 Outside Delhi Set III
Note: Except for the following questions, all the remaining questions have been asked in previous sets.
Question 1.
What are n-type semiconductors? [1]
Question 4.
What is the basicity of H3PO2 acid and why? [1]
Question 5.
Write the IUPAC name of the following: [1]
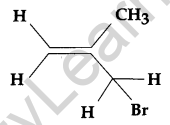
Answer:
3 Bromo-2-methyl propene.
Question 7.
Write a reaction which shows that all the carbon atoms in glucose are linked in a straight chain. [1]
Answer:
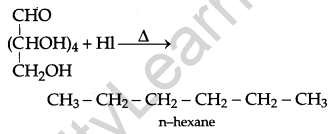
Question 8.
What is the cause of a feeling of depression in human beings? Name a drug which can be useful in treating this depression. [1]
Answer:
The inability to achieve one’s goal and extra work may cause the level of noradrenaline low, as a result, the signal-sending activity becomes low and the person suffers from depression. Tranquilizers such as Equanil can help in treating depression.
Question 11.
Explain the role of each of the following: [2]
(i) NaCN in the extraction of silver.
(ii) SiO2 in the extraction of copper.
Answer:
(i) Dil. NaCN solution is used to leach silver (Ag) from silver ore in the presence of air. The silver metal is obtained by replacement.
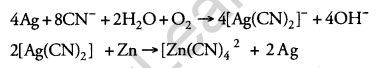
(ii) SiO2 is used to remove impurity and it reacts with FeO to form easily removable feasible slag
![]()
Question 22.
Write three distinct features of chemisorption which are not found in physisorption. [3]
Answer:
The three distinct features of chemisorption are:
- It is irreversible.
- It occurs by the chemical bond formation and hence requires activation energy.
- It is highly specific in nature.
Question 23.
Explain each of the following observations: [3]
(i) With the same d-orbital configuration (d4), Cr2+ is a reducing agent while Mn3+ is an oxidising agent.
(ii) Actinoids exhibit a much larger number of oxidation states than the lanthanoids.
(iii) There is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals.
Answer:
(i) Cr2+ is reducing because when it loses one electron to form Cr3+: [Ar]3d3, it has three unpaired electrons in lower energy d-orbitals which are more stable whereas M3+ is oxidizing because after gaining one electron it becomes Mn2+ which has more stable electronic configuration due to half-filled d-orbitals Mn2+:[Ar]3d5
(ii) Due to comparable energies of 5f, 6d and 7s orbitals and unpaired electrons in these orbitals, actinoids exhibit a much larger number of oxidation states than the lanthanoids.
(iii) Because along with transition series, nuclear charge increases which tend to decrease the size but the addition of electrons in the penultimate d-subshell increases the screening effect which counters balances the effect of increased nuclear charge. Thus, atomic radii do not change.
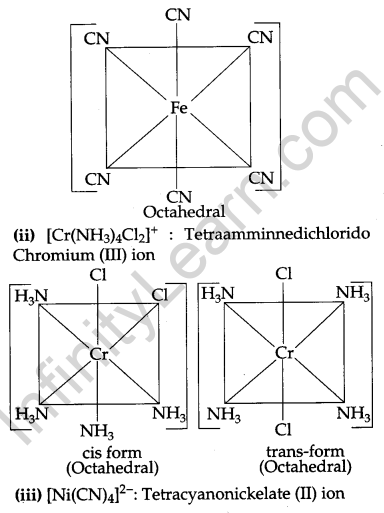
Question 24.
Name the following coordination entities and describe their structures: [3]
(i) [Fe(CN)6]4-
(ii) [Cr(NH3)4Cl2]+
(iii) [Ni(CN)4]2-
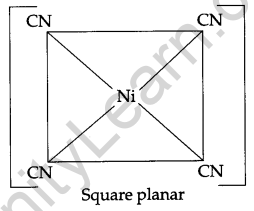
(Atomic Numbers Fe = 26, Cr = 24, Ni = 28)
Answer:
(i) [Fe(CN)6]4-: Hexacyanoferrate (II) ion.
Question 26.
Define the following as related to proteins: [3]
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Answer:
(i) Peptide linkage is the amide linkage between two amino acids to form proteins and polypeptides. CO-NH is a peptide linkage.
(ii) The simple linear structure of a protein molecule in a specific sequence in which various amino acids are present.
(iii) When a protein in its native form is subjected to any physical change like change in temperature or chemical change like change in pH, the H-bonds gets disturbed. Due to this globules unfold and helix gets uncoiled and protein loses its biological activity. This is called denaturation of the protein.






