To Determine the Surface Tension of Water by Capillary Rise Method
Aim
To determine the surface tension of water by capillary rise method.
Apparatus
Three capillary tubes of different radii and a tipped pointer clamped in a metallic plate with a handle, travelling microscope, clamp and stand, a fine motion adjustable height stand, a flat bottom open dish, clean water in a beaker, thermometer.
Theory

Diagrams
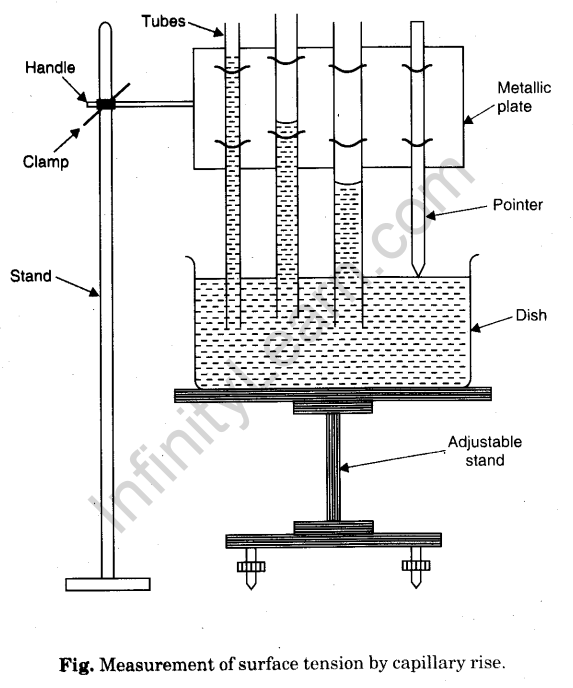
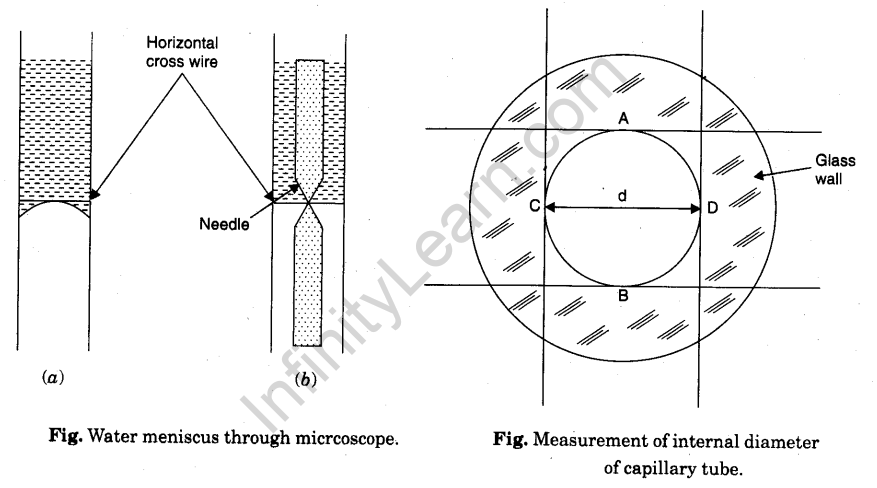
Procedure
(a) Setting the apparatus
1. Place the adjustable height stand on the table and make its base horizontal by level-ling screws.
2. Take dirt and grease free water in an open dish with flat bottom and put it on the top of the stand.
3. Take three capillary tubes of different radii (ranging from 0.05 mm to 0.15 mm).
4. Clean and dry them, clamp the capillary tubes in a metallic plate in order of increasing radius. Also clamp a pointer after third capillary tube.
5. Clamp the horizontal handle of the metallic plate in a vertical stand, so that the capillary tubes and the pointer become vertical.
6. So adjust the height of metallic plate that the capillary tubes dip in water in open dish.
7. Adjust the position of the pointer, such that its tip just touches the water surface.
(b) Measurement of capillary rise
8. Find the least count of the travelling microscope for the horizontal and the vertical scale. Record the same in the note-book.
9. Raise the microscope to a suitable height, keeping its axis horizontal and pointed towards the capillary tubes.
10. Bring the microscope in front of first capillary tube (which has maximum rise).
11. Make the horizontal cross wire just touch the central part of the concave meniscus seen convex through microscope.
12. Note the reading of the position of the microscope on the vertical scale.
13. Now move the microscope horizontally and bring it in front of the second capillary tube.
14. Lower the microscope and repeat steps 11 and 12.
15. Repeat steps 11 and 12 for third capillary tube.
16. Lower the stand so that pointer tip becomes visible.
17. Move the microscope horizontally and bring it in front of the pointer.
18. Lower the microscope and make the horizontal cross wire touch the tip of the pointer. Repeat step 12.
(c) Measurement of the internal diameter of the capillary tube
19. Place the first capillary tube horizontally on the adjustable stand.
20. Focus the microscope on the end dipped in water. A white circle (inner bore) surrounded by a green circular strip (glass cross section) will be seen.
21. Make horizontal cross-wire touch the inner circle at A. Note microscope reading on vertical scale.
22. Raise the microscope to make the horizontal cross-wire touch the circle at B. Note the reading (the difference gives the vertical internal diameter AB of the capillary tube).
23. Move the microscope on horizontal scale and make the vertical cross wire touch the inner circle at C. Note microscope reading on horizontal scale.
24. Move the microscope to the right to make the vertical cross-wire touch the circle at D. Note the reading (the difference gives the horizontal internal diameter CD of the capillary tube).
25. Repeat steps 19 to 24 for other two capillary tubes.
26. Note temperature of water in dish.
27. Record your observations as given ahead.
Observations
Least count of travelling microscope (L.C.) =………..cm.
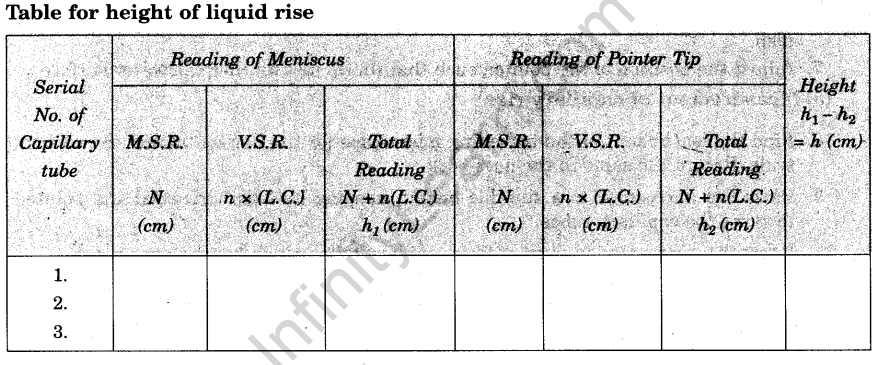
Calculations
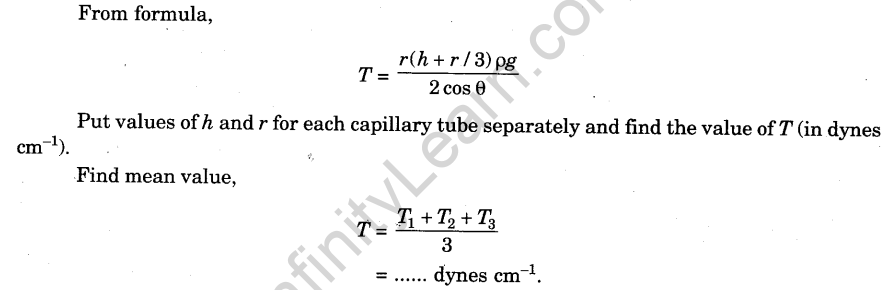
Result
The surface tension of water at t°C = …….. dynes cm-1.
Precautions
- Capillary tube and water should be free from grease.
- Capillary tube should be set vertical.
- Microscope should be moved in lower direction only to avoid back lash error.
- Internal diameter of capillary tube should be measured in two mutually perpendicular directions.
- Temperature of water should be noted.
Sources of error
Water and capillary tube may not be free from grease.
Viva Voce
Question. 1. Give symbol and unit of surface tension.
Answer. The symbol is T and S.I. unit is N m-1.
Question. 2. Give relation between surface tension and surface energy.
Answer. Surface energy = Surface tension x Change in area.
Question. 3. Why liquids surfaces are generally curved ?
Answer. It is due to surface tension.
Question. 4. Why is there a pressure difference on the two sides of a curved liquid surface ?
Answer. Curved free liquid surface has more area. It tends to become flat to minimise the surface area (property of surface tension). Hence pressure is not the same above and below the surface.
Question. 5. On which side of the liquid surface, pressure is more ?
Answer. Pressure is more on the concave side of the free liquid surface.
Question.6. How much is the excess pressure inside a spherical liquid drop and a spherical liquid bubble?
Answer.

Question.7. What is a capillary ?
Answer. It is an open ended tube with very uniform fine bore.
Question.8.Why the liquid should be free from grease ?
Answer. Grease reduces surface tension of liquid.
Question.9.Do all liquids rise in a capillary tube ?
Answer. No, liquids which make a convex meniscus in capillary tube, are depressed. Mercury is depressed in glass capillary tube.
Question.10.What will happen if the length of the capillary tube is less than the height up to which liquid would rise in it ? Will the liquid overflow ?
Answer. No, the liquid will not overflow. The liquid will rise up to the upper edge of the capillary tube. Then its surface will become less curved and become plane.
Therefore, radius of curvature (R) increases.

Question.11. Why do we prefer fresh tap water rather than pure distilled water for determination of surface tension ?
Answer. Distilled water is slightly greasy and its surface tension will be less.
Question.12. What is surface tension of water ?
Answer. It is 7.275 x 10-2 N-m-1 at 20°C.
Question.13. Why do you measure the internal diameter of the capillary tube in two mutually perpendicular directions ?
Answer. It is done to take the mean to eliminate the error if the bore is not circular.




