NCERT Solutions for Class 7 Science Chapter 6 Physical and Chemical Changes
Subject specialists have designed NCERT solutions for Science Class 7 Chapter 6 which includes thorough solutions for reference. These solutions are updated according to the latest CBSE syllabus for 2012-22 and are provided in easy language for understanding. Tips and tricks are also provided.
These solutions are provided so a student can clear his doubts and get help with deep understanding of the concept. Also you can refer them to make the chapter notes and revisions notes. PDF of this can also be downloaded from website.
Classify the changes involved in the following processes as a physical or chemical change ?
a) Photosynthesis b) Dissolving sugar in water c) Burning of coal
d) Melting of wax e) Beating Aluminium to make aluminium foil f) Digestion of food
Ans : a) Chemical change b) Physical change
c) Chemical change d) Physical change
e) Physical change f) Chemical change
2. State whether the following statements are true or false. In case a statement is false write the correct statement ?
a) Cutting a log of wood into pieces is a chemical change
b) Formation of manure from leaves is a physical change
c) Iron pipes coated with Zinc do not get rusted easily
d) Iron and rust are the same substances.
e) Condensation of steam is not a chemical change.
Ans : a) False :
Correct statement : cutting a log of wood into pieces is a physical change.
b) False :
Correct statement : Formation of manure from leaves is a chemical change.
c) True
d) False
Correct statement : Iron and rust are two different substances.
e) True
3. Fill in the blanks in the following statements
a) When Carbon dioxide is passed through lime water, it turns milky due to the formation of ________
b) The chemical name of baking soda is _________
c) Two methods by which rusting of Iron can be prevented are _________ and _________
d) Changes in which only _________ properties of a substance are Altered are called physical changes.
e) Change in which new substances are formed are called _______ changes.
Ans : a) Calcium carbonate b) Sodium hydrogen carbonate (Sodium bicarbonate)
c) Painting or greasing, Galvanization d) Physical e) Chemical
4. When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it ? Explain ?
Ans : The reaction between baking soda and lemon juice can be given as below.
Lemon Juice + Baking Soda Carbon dioxide + other substances
(citric acid) (Sodium bicarbonate) (Bubbles)
It is a chemical change
5. When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both physical and chemical changes take place ?
Ans : When a candle burns, both physical and chemical changes occur.
i) Melting of wax, vapourisation of melted wax are physical changes
ii) Burning of wax to give CO2, heat and light are chemical changes.
Another example in which physical and chemical changes occur is L.P.G.
6. How would you show that setting of curd is a chemical change ?
Ans : We can say that setting of curd is a chemical change because we can not get back the original substance i,e. milk and curd is a new substance is formed with different taste, smell and other properties. During this process Lactose is converted into Lactic acid.
7. Explain why burning of wood and cutting it into small pieces are considered as two different types of changes ?
Ans : Burning of wood is a chemical change because on burning new substances are formed as follows.
wood + Oxygen charcoal + CO2 + heat + light
Cutting of wood into small pieces is physical change because no new substance is formed. We can only reduce the size of wood.
8. Describe how crystals of Copper Sulphate are formed?
Ans :
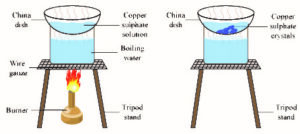
Take 100 ml of water in a clean and dry 250ml beaker and add few drops of dil . H2SO4and heat the contents of the beaker, then add powdered CuSO4 to the boiling acidulated water slowly until saturation. Now filter the solution and allow it for cooling without disturbing the beaker. After some time CuSO4 crystals are formed at the bottom of the beaker.
9. Explain how painting of an iron gate prevents it from rusting ?
Ans : It is known that for rusting the presence of Oxygen and moisture are essential. Painting prevents the contact of Iron with oxygen and moisture and prevents the rusting of iron gate.
10. Explain why rusting of iron objects is faster in coastal areas than in deserts ?
Ans : Content of moisture in the air in coastal areas is higher than the air in deserts. So the process of rusting is faster in coastal areas.
11. The gas we use in the kitchen is called liquified petroleum gas (L P G). In the cylinder it exist as a liquid. When it comes out from the cylinder it becomes a gas (change – A) then it burns (change – B). The following statements pertain to these changes. choose the correct one?

i) Process – A is a chemical change.
ii) Process – B is a chemical change.
iii) Both processes A and B are chemical changes.
iv) None of these processes is a chemical change.
Ans : ii) Process – B is chemical change.
12. Anaerobic bacteria digest animal waste and produce biogas (Change – A). The biogas is then burnt as fuel (Change – B) the following statements pertain to these changes. choose the correct one.
i) Process – A is a chemical change
ii) Process – B is a chemical change
iii) Both processes A and B are chemical changes.
iv) None of these processes is a chemical change.
Ans :

iii) Both processes A and B are chemical changes.
Class 7 Science NCERT Solutions for Chapter 6 – Physical and Chemical Changes
This chapter deal the Physical and Chemical changes through various activities. After this it talks about topic, rusting of iron and crystallisation. Infinite learn is the best source of NCERT Solutions for this chapter as it includes all the topic covered in NCERT Textbook
Important topics covered in NCERT Solutions of this Chapter
- Physical Changes
- Chemical Changes
- Rusting of Iron
- Crystallisation
Further, the NCERT Solutions are also present in PDF format and students can directly assess these materials online or offline, by visiting our website of Infinite learn.
About Infinite Learn Solutions
Infinite Learn mission is to create world-class learning content for students. We are focussed on raising the students learning abilities. These Solutions have been structured very carefully to give several benefits to the students that utilize these study materials. That is why, these are crafted by subject matter experts to focus on concept and its explanation.
For any type of support related to the NCERT Solutions for Class 7 given here, students can approach our support team who will help clear all their doubts. Students can also check out Infinite Learn for a more efficient learning experience.
Frequently Asked Questions on NCERT Solutions of this Chapter
How many questions are present in these NCERT Solutions for Class 7 Science?
About 12 questions are available in these NCERT Solutions. These questions cover short answer questions and MCQs according to the latest NCERT textbook. The NCERT Solutions at Infinite learn is used to know about the method of answering complex questions. The solutions are provided by subject matter experts after taking a vast research on each concept. Students can cross check their answers and get their doubts cleared.
What are the important topics covered in these NCERT Solutions?
The important topics are –
1. Physical Changes
2. Chemical Changes
3.Rusting of Iron
4. Crystallisation
Why should we download NCERT Solutions for Class 7 Science Chapter 6 from Infinite learn?
At INFINITE LEARN you can get PDF of accurate solutions of this chapter. The NCERT Textbook Solutions for this chapter have been formulated by mathematics experts at INFINITE LEARN. All these solutions are accordance to the new pattern of CBSE, so the students can be ready for the exams.


