Table of Contents
Subject – CBSE Class 9 Science
Year of Examination – 2019.
Time Allowed: 3 hours
(GENERAL INSTRUCTIONS)
(i) The question paper comprises of two sections. A and 13. You are to attempt both the sections.
(ii) All questions are compulsory. However, internal choice has been provided in two questions of three marks each and one. question office marks. Only one option in. such questions is to be attempted.
(iii) All questions of section A and all questions of section B are to be attempted separately.
(iv) Question numbers 1 and 2 in section A are one mark questions. These are to be answered in one word or in one sentence.
(v) Question numbers 3 to 5 in section A are two marks questions. These are to be answered in about 30 words each.,
(vi) Question numbers 6 to 15 in section A are three marks questions. These are to be answered in about 50 words each..
(vii) Question numbers 16 to 21 in section A are five marks questions. These arc to be answered in about 70 words each.
(viii) Question numbers 22 to 27 in Section B are two marks questions based on practical skills. These are to he answered in brief.
SECTION – A
Question 1:
Give a relation correlating sound velocity with its frequency and wave-length. Answer:
Velocity of sound (υ) = frequency (ν) x wavelength (λ).
Question 2:
Suggest separation techniques one would need to employ to separate the following mixtures:
(a) Mercury and water
(b) Potassium Chloride and ammonium chloride.
Answer:
(a) Using separating funnel.
(b) Using sublimation.
Question 3:
Liquids like ether and acetone are kept in cool places. Why ?
Answer:
Ether and acetone have low boiling temperature and are highly volatile. They are kept in cool places. If they are not kept in cool places :
(i) these liquids will evaporate very fast,
(ii) the pressure inside the bottle may increase due to which the bottle may break.
Question 4:
Draw a well-labelled diagram of a prokaryotic cell. How can you identify it as prokaryotic cell ?
Answer:

It is a prokaryotic cell as it lacks membrane bound cell organelles.
Question 5:
Differentiate between a tracheid and a vessel.
Answer:

Question 6:
If you are trying to push a heavy box on a horizontal surface, list various forces on the box. State the condition under which this box will start sliding on the surface. How will the magnitude of applied force required to move the box change if:
(i) weight of the box is increased,
(ii) the surface on which the box is placed is made more rough ?
Answer:
Various forces acting on the box are :
(i) Its weight ‘W’ acting vertically downward,
(ii) reaction force ‘R’ due to horizontal surface,
(iii) force of push ‘F’ and
(iv) frictional force ‘f. Forces have been shown in figure.
The box will start sliding on the surface only if the pushing force F is greater than the frictional force f i.e., F > f.
(i) If weight of the box is increased, the magnitude of applied force must increase.
(ii) If the surface is made more rough, the magnitude of applied force must increase.

Question 7:
The velocity-time graph of a car of 1000 kg mass is given alongside. From the graph
answer
the following :
(a) When is the maximum force acting on the car ? Why?
(b) What is the retarding force ?
(c) For how long is there no force acting ?
Answer:
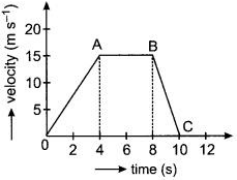
(a) In the u-t graph the OA part represents uniformly
accelerated motion with an acceleration \(a=\frac { 15-0 }{ 4 } =3.75m{ s }^{ -2 }\)
∴Force in this region is maximum having a value
F = ma = 1000 × 3.75 = 3750 N
(b) In the u-t graph the part BC represents uniformly retarded motion with an acceleration
\(a=\frac { 0-15 }{ 10-8 } =-7.5m{ s }^{ -2 }\)
∴Retarding force F = ma = 1000 × (- 7.5) = – 7500 N
(c) The region AB of the graph represents uniform motion with a constant velocity of 15 m s-1.Hence there is no force acting on the car during the part AB from 4 s to 8 s.
Question 8:
(a) Distinguish between G and g.
(b) Relative density of steel is 7.8. What is its density ?
Answer:
(a)
|
Gravitational constant G |
Acceleration due to gravity g |
|
(1) It is defined as the force of attraction between two objects of unit mass each separated by unit distance. (2) It is a universal constant and its value is 6.678 x 10 11 N m2 kg-2, |
(1) It is defined as the acceleration of an object freely falling under the action of force or gravity. (2) It. is a constant at a given place and its value changes from place to place. Mean value of g on surface of earth is 9,8 m s-2. |
(b) Density of steel = relative density of steel × density of water = 7.8 × 103 kg m-3.
Question 9:
(a) Distinguish between loudness and intensity of sound.
(b) What is the audible range of the average human ear ?
Answer:
(a)
|
Loudness of sound |
Intensity of sound |
|
(i) Loudness of sound is a measure of the response of the ear to the sound, whether it is loud or soft. |
(i) Intensity is a physical quantity and its magnitude does not depend on the sensitivity of the ear etc. |
|
(ii) Loudness depends on the sensitivity of ear, (iii) Its unit is decibel. |
(ii) Intensity of sound is the amount of sound energy passing per second through unit area. (iii) Its unit is W m-2. |
(b) For an average human ear the audible range extends from 20 Hz to 20000 Hz (or 20 kHz).
Question 10:
Clothes get dry faster in summers than in winters. Give reason.
Answer:
In summer, the temperature is higher. More water molecules get the higher kinetic energy, leave the surface of clothes and move to the atmosphere. Therefore clothes get dry faster in summer.
Question 11:
Describe an activity to show that particles of matter have spaces between them.
Answer:
The activity is performed as under:
(i) Take a 100 ml_ beaker.
(ii) Fill half of the beaker with water and mark the level of water with a pen.
(iii) Dissolve about 5 g of salt into water with the help of a glass rod.
(iv) Observe the change in level of water.
We find that there is no change in the level of water in the beaker. That means the particles of salt have occupied the empty spaces between particles of water, so no change in water level has taken place.,
The activity proves that particles of matter have empty spaces between them.
OR
Identify solute and solvent in the following solutions : aerated drinks, tincture of iodine, lemon water.
Answer:
Aerated drinks like soda water are solutions of carbon dioxide gas in water. Hence, soft drinks contain CO2 as solute and water as solvent.
Tincture of iodine is a solution of iodine in alcohol. It has iodine (solid) as solute and alcohol (liquid) as the solvent.
Lemon water is a solution of lemon juice in water. So, it has lemon juice as solute and water as solvent.
Question 12:
(a) Why do Sclerenchyma cells have a narrow lumen ?
(b) Where are these tissues present and why ?
Answer:
(a) Sclerenchyma cells have narrow lumen as they have thick and lignified cell walls.
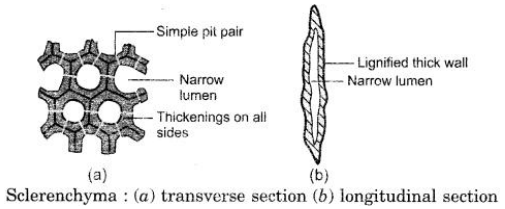
(b) Since these provide mechanical support and enables plants to bear various stresses, hence is found in parts that provide support like stem, petiole, pedicel etc.
OR
(a) What is Haversian canal system ?
(b) With which animal tissue is this system associated ?
(c) Draw a neat diagram to exhibit Haversian canal system and tell about its significance.
Answer:
(a) A haversian canal system comprises of nutrient filled canals around which bone cells or osteocytes occur in concentric rings or lamellae. These canals have 1-2 blood capillaries, nerve fibres and connective tissue.
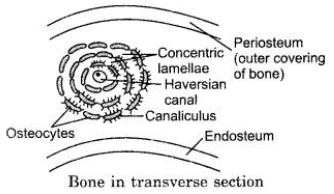
(b) Haversian canal system are associated with bone, a type of connective tissue.
(c) The bony endoskeleton forms the supporting framework of the body which provides protection to vital organs like brain, heart, lungs etc. Bones form various types of joints which take part in body movements including locomotion. Bone is a reservoir of calcium, phosphorus and other minerals.
Blood cells are formed in red bone marrow of the bones.
Question 13:
State the conditions essential for production of best quality of honey. Name a product other than honey which is obtained through bee keeping.
Answer:
Tb obtain good quality and high yield of honey, beehive should be developed near pasturage. The selected honey bees should show less swarming. They should be protected from diseases and pests.
Bees wax is another important product obtained through bee keeping.
Question 14:
What do you know about hole in ozone layer ? Explain the probable damages caused by it.
Answer:
Ozone, 03 is a molecule that contains three atoms of oxygen. Ozone is poisonous but it is not stable near the Earth’s surface. It occurs in the zone of Earth’s atmosphere called stratosphere.
Ozone absorbs harmful ultraviolet radiations from the Sun. Thus it protects us from certain diseases like cancer.
Various man-made compounds like chlorofluorocarbons (CFCs) react with ozone molecule and cause depletion in the ozone layer in the atmosphere.
Recently a hole in the ozone layer has been discovered above the Antarctica. Due to ozone layer depletion, UV-rays can enter the Earth’s atmosphere and cause certain diseases.
Question 15:
Kishore started helping his parents in the fields. He told his father that the soil forms the most important reservoir of plant nutrients. So, to obtain high yield soil fertility needs to be maintained. One of the ways he suggested was growing leguminous crops.
(i) How many plant nutrients are provided by the soil ? And how are these nutrients replenished in the soil ?
(ii) Why did Kishore suggest growing legumes in the field ?
(iii) List the two values that motivated Kishore to help his parents.
Answer:
(i) There are sixteen nutrients essential for plants. Soil supplies thirteen nutrients to plants. Six nutrients which are required in large quantities are called macro or major nutrients. The remaining seven nutrients which are required by plants in small quantities are called micro or minor nutrients.
Soil can be enriched by supplying these nutrients from external sources as manures and fertilisers.
(ii) Since legumes are capable of fixing atmospheric nitrogen with the help of Rhizobium, they enrich the soil in N2 content. Hence Kishore suggested growing legumes.
(iii) His concern for his parents’ well being, him being environmentally conscious and concerned.
Question 16:
(a) Which of the two decides the direction of motion of an object: its velocity or the acceleration acting on it ? Explain by giving an example.
(b) A motorcyclist riding motorcycle A, who is travelling at 36 km h’1, applies the brakes and stops the motorcycle in 10 s. Another motorcyclist of motorcycle B, who is travelling at 18 km h’1, applies the brakes and stops the motorcycle in 20 s. Plot speed-time graphs for the two motorcycles. Which of the two motorcycles travelled farther before it came to a stop ?
Answer:
(a) It is the direction of velocity of an object which determines the direction of motion of a particle. As an example, let us consider a ball moving in vertically upward direction when projected upward with a finite velocity. In this case, an acceleration due to gravity is acting on the ball in vertically downward direction. However, the direction of motion is vertically upward which is same as the direction of velocity.

OR
(i) When can we say the motion of an object as uniform motion ? What can be the shape of the path covered by a moving object to have uniform speed and uniform velocity ?
(ii) Study the velocity-time graph of figure and calculate :
(a) the acceleration from A to B.
(b) the acceleration from B to C.
(c) the distance covered in the region ABD.
(d) the average velocity from C to D.
(e) the distance covered in the region BCFE.
Answer:
(i) The motion of an object is said to be uniform, if it covers equal displacements in equal intervals of time.
If a moving object is to have uniform speed and uniform velocity then its path must be a straight line path along a given direction.
(ii) (a) For region AB of graph, u = 0, v = 25 m s_1 and t = 3 s
Question 17:
(a) Define kinetic energy. Obtain an expression for the kinetic energy of an object.
(b) A ball of mass 400 g rolls on a ground with a uniform speed of 25 m s’1. Find the kinetic energy possessed by it.
Answer:
(a) Kinetic energy of an object is the energy possessed by it by virtue of its state of motion. Every moving object possesses kinetic energy.
Consider an object of mass m in a state of motion with an initial velocity u. Let now a constant force
Facts on it and displaces the body through a distance s in the direction of force applied.
Work done on the object W = Fs
Due to the work done on the body, let velocity of the object changes from u to v and a be the acceleration produced.

If the object started from rest, then u = 0 and hence W = 1/2 mv2
The work done on the object is equal to the kinetic energy imparted to the object.
Thus, the kinetic energy possessed by an object of mass rn moving with a uniform
velocity v is given by
Ek = 1/2 mv2
(b) Here mass of ball m = 400 g = 0.4 kg and speed of ball v = 25 m s-1.
∴ Kinetic energy of ball Ek = 1/2 mv2 = 1/2 × 0.4 × (25)2 = 125 J
Question 18:
(a) Write chemical formulae of all the compounds that can be formed by the combination of following ions :
Ca2+, K+ Fe3+, Cl–, SO42-
(b) Molar mass of nitrogen is 14 u. What will be the mass of one atom of nitrogen in grams ?
Answer:
(a) Compounds of Ca2+
CaCl2
CaSO4
Compounds of K+
KCl
K2SO4
Compounds of Fe3+
FeCl3
Fe2(SO4)3
(b) Molar mass of nitrogen is 14 u
6.022 × 1023 atoms of nitrogen weigh = 14 g
![]()
Question 19:
(a) The following data represents the distribution of electrons, protons and neutrons in atoms of four elements A, B, C, D.
|
Element |
Protons | Neutrons |
Electrons |
|
A |
10 |
10 |
10 |
Answer the following questions :
(i) Write the electronic distribution in atoms of elements A and D.
(ii) Element A is an inert gas. why ?
(iii) What is the valency of element C ?
(b) The average atomic mass of a sample of element X is 16.2 u. What are the percentages of isotopes of 16Xs and 18Xs respectively ?
Answer:
(a) (i) Electronic distribution in Element A = 2, 8 Element B = 2, 8, 3
(ii) Element A is an inert gas because it has 8 electrons in the outermost shell.
(iii) The element C has electronic configuration 2, 8, 2. Hence, valency of C is 2.
(b) Suppose the percentage of 16X8 in the mixture is A. Then percentage of 18X8 = 100

Question 20:
Complete the following flow chat:

Name one or more types of connective tissues.
Answer:
(a) – Connective (b) – Plasma (c) – RBC
(d) – Blood platelets (e) -Neutrophil (f) – Eosinophil
(g) – Basophil (h) -Lymphocyte (i) – Monocyte
Other connective tissues are areolar, adipose, tendon, ligaments, bones, cartilage and lymph.
Question 21:
Draw a neat and labelled diagram to illustrate nitrogen cycle. Mention the main steps involved in this process.
Answer:

Nitrogen fixation involves the following steps:
(i) Conversion of atmospheric nitrogen into nitrates and nitrites by nitrogen fixing bacteria which are found in the roots of legumes (plants which give us pulses) or during lightning in the sky.
(ii) Formation of proteins by plants and animals from nitrates.
(iii) On decay and death of plants and animals, denitrifying bacteria convert the nitrates back to elemental nitrogen.
SECTION – B
Question 22:
State two precautions that should be observed while making use of an overflow can, Answer:
Two important precautions which should be observed while making use of an overflow can are as follows :
(i) The overflow can should be filled with water until the water begins to flow from its spout. Wait till the last drop of excess wrater flows out. This is to ensure that the level of water in the can is up to its brim.
(ii) When the given solid object is lowered in the overflow can, the object should not touch the walls or the base of the overflow can.
Question 23:
Would you prefer amplitude of the pulse to be small or large ? Why ?
Answer:
We prefer to keep the amplitude of the pulse appreciably large so that it can get reflected sufficient number of times at the fixed ends of the string/slinky before fading out. In this way, total length covered by the pulse is large and correspondingly time taken by pulse to cover this journey is sufficient and can be measured easily using a stop clock.
Question 24:
Dipti was asked to prepare three separate solutions in three beakers A, B and C by mixing sugar, fine sand and starch respectively in water. Tell which of the solutions are stable and which are unstable ?
Answer:
Beaker A – Stable Beaker B – Unstable Beaker C – Stable –
Question 25:
A mixture of iron filings and sulphur was heated in a china dish as shown in the figure.
(a) Give the formula of the product and the reaction involved.
(b) What is the colour of the product ?

Answer:
(a) The formula of the product is FeS. The reaction is represented as under:
Fe + S —–> FeS
(b) The product has a black colour.
Question 26:
Without uprooting a dicot plant, list any two identifying observable features of the same. Also give two examples of dicot plants.
Answer:
The observable identifying features of dicots are as follows :
(i) Leaves of dicots exhibit reticulate venation.
(ii) Presence of pentamerous flowers.
(iii) Stem is hard, brown and woody due to secondary growth.
(iv) Seeds of dicot plants can be split into two i.e., they have two cotyledons.
Question 27:
A student was asked to prepare a temporary mount of cheek cells which he did but his slide got mixed up with the slide prepared by another student. The other student had prepared a temporary mount of onion peel. Which of the following observation will help him in identifying his slide of cheek cells ?
Answer:
The student can recognise his slide of cheek cell from the slide prepared by another student in following ways :
(i) Cheek cells are stained by a chemical called methylene blue which colours the tissue blue.
(ii) Cheek cells are irregular shaped polygonal cells.
(iii) Cheek cells do not have a rigid cell wall.
We hope the Solved CBSE Sample Papers for Class 9 Science Set 4, help you. If you have any query regarding CBSE Sample Papers for Class 9 Science Solved Set 4, drop a comment below and we will get back to you at the earliest.


