Table of Contents
Ammonia is a compound that is widely known and used in various applications. Its chemical formula is NH3, which represents its composition of one nitrogen (N) atom bonded to three hydrogen (H) atoms.
Formula and Structure of Ammonia
It has a characteristic trigonal pyramidal molecular shape, where the nitrogen atom occupies the central position, and the three hydrogen atoms are arranged symmetrically around it.
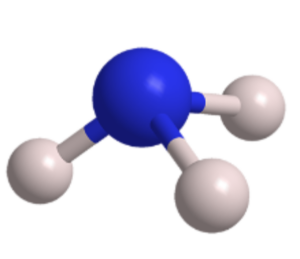
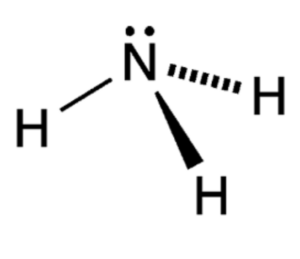
Ammonia (NH3)
The nitrogen atom forms three sigma bonds with the three hydrogen atoms, resulting in a stable and covalent structure.
the nitrogen atom acquires a partial negative charge (δ-) while the hydrogen atoms develop partial positive charges (δ+). This polarity gives ammonia its characteristic properties, such as its ability to act as a weak base and form hydrogen bonds.
Preparation of Ammonia
Ammonia is prepared by the Haber process on an industrial scale. One mole of Nitrogen gas is made to react with 3 moles of hydrogen gas at high pressure and 400-550℃. Metal catalysts are often used to catalyze the reaction. The chemical reaction known as the ammonia equation is as shown below:
N2 + 3H2 → 2NH3
This reaction yields ammonia gas which has a foul odour and is transparent in nature. It can be liquified at a very low temperature (-196℃), which is known as liquid Nitrogen. Liquid Nitrogen is the most commonly used solvent in laboratories. Ammonia acts as a very good base.
Ammonia Physical Properties
Ammonia is a colourless gas with a pungent smell.
Ammonia (NH3) exhibits several physical properties that are important to understand. Here are some key physical properties of ammonia:
- State: At standard temperature and pressure (STP), ammonia is a colorless gas. However, it can be easily liquefied under moderate pressure or cooled to form a solid.
- Odour: Ammonia has a distinctive pungent smell often associated with cleaning products or urine. Even at low concentrations, it can be easily detected by the human nose.
- Density: The density of ammonia gas is lower than that of air. It has a density of approximately 0.7713 grams per liter (g/L) at STP. Ammonia gas is lighter than air and tends to rise.
- Boiling Point: The boiling point of ammonia is -33.34 degrees Celsius (-28.012 degrees Fahrenheit) at atmospheric pressure. This low boiling point allows ammonia to readily vaporize, making it useful as a refrigerant.
- Melting Point: Ammonia can be solidified into a white crystalline solid at temperatures below -77.73 degrees Celsius (-107.914 degrees Fahrenheit). The solid form of ammonia is often referred to as “ammonia snow.”
- Solubility: Ammonia is highly soluble in water, forming a solution known as ammonium hydroxide. The solubility of ammonia in water increases with decreasing temperature and increasing pressure.
- Vapour Pressure: Ammonia has a relatively high vapour pressure, which means it readily evaporates into the gas phase at normal temperatures. This property contributes to its strong odour and volatility.
- Thermal Conductivity: Ammonia is a good conductor of heat. It has a high thermal conductivity, which makes it useful in various heat transfer applications.
- Flammability: Ammonia itself is not flammable, but it can support the combustion of other substances. It acts as a fuel by providing nitrogen and hydrogen atoms necessary for combustion reactions.
- pH and Alkalinity: When dissolved in water, ammonia forms an alkaline solution. It reacts with water molecules to produce hydroxide ions (OH-), resulting in an increase in pH.
Chemical Properties of Ammonia
Ammonia (NH3) exhibits several chemical properties that are important to understand. Here are some key chemical properties of ammonia:
- Basicity: Ammonia is a weak base, meaning it has the ability to accept a proton (H+) from an acid. It can react with acids to form ammonium salts. For example, with hydrochloric acid (HCl), ammonia reacts to form ammonium chloride (NH4Cl):
NH3 + HCl → NH4Cl
- Reducing Agent: Ammonia can act as a reducing agent, meaning it has the ability to donate electrons and undergo oxidation itself. It is capable of reducing certain metal ions, such as silver (Ag) and copper (Cu), to their respective elemental forms.
- Reaction with Acids: Ammonia readily reacts with acids to form salts. The resulting compounds are called ammonium salts, where the ammonia molecule donates a lone pair of electrons to the proton from the acid. This reaction is known as neutralization.
- Reaction with Halogens: Ammonia reacts with halogens (such as chlorine, bromine, and iodine) to form compounds known as ammonium halides. For example, with chlorine gas (Cl2), ammonia reacts to form ammonium chloride (NH4Cl):
NH3 + Cl2 → NH4Cl
- Complex Formation: Ammonia has the ability to form coordination complexes with transition metal ions. These complexes are formed due to the coordination of the lone pair of electrons on the nitrogen atom with the metal ion. Ammonia is commonly used as a ligand in coordination chemistry.
- Combustibility: While ammonia itself is not flammable, it can support the combustion of other substances. It can act as a fuel by providing nitrogen and hydrogen atoms necessary for combustion reactions.
- Alkaline Solution Formation: When dissolved in water, ammonia forms an alkaline solution. It reacts with water molecules to produce ammonium ions (NH4+) and hydroxide ions (OH–). This gives the solution its characteristic basic properties.
It is important to note that ammonia has a strong pungent odour and can be toxic in high concentrations. Care should be taken when handling and using ammonia to ensure safety.
Ammonia Uses
Ammonia is commonly used in various industrial and household applications. It serves as a vital ingredient in the production of fertilizers, where it provides a source of nitrogen necessary for plant growth. It is also used in the manufacturing of various chemicals, such as plastics, dyes, and explosives.
In addition to its industrial uses, ammonia finds application as a cleaning agent and a refrigerant. It is an efficient cleaner due to its basic properties, allowing it to react with acidic substances. As a refrigerant, ammonia has excellent heat transfer capabilities and is environmentally friendly compared to certain synthetic refrigerants.
While ammonia is essential for many purposes, it should be handled with care. It is highly toxic when inhaled in high concentrations and can cause severe irritation to the respiratory system. Precautions should be taken to ensure its safe storage, handling, and use in industrial and household settings.
Solved Examples on Ammonia Formula
Example 1: Ammonia (NH3) reacts with oxygen (O2) to produce nitrogen monoxide (NO) and water (H2O). If 10 moles of ammonia react with excess oxygen, how many moles of nitrogen monoxide will be produced?
Solution:
The balanced chemical equation for the reaction between ammonia and oxygen is:
4NH3 + 5O2 → 4NO + 6H2O
From the equation, we can see that 4 moles of ammonia react to produce 4 moles of nitrogen monoxide. Therefore, we need to determine the number of moles of nitrogen monoxide produced when 10 moles of ammonia react.
Using the ratio from the balanced equation, we can set up the following proportion:
4 moles of ammonia / 4 moles of nitrogen monoxide = 10 moles of ammonia / x moles of nitrogen monoxide
Cross-multiplying and solving for x, we get:
4 moles of ammonia * x moles of nitrogen monoxide = 10 moles of ammonia * 4 moles of nitrogen monoxide
4x = 40
x = 40 / 4
x = 10
Therefore, when 10 moles of ammonia react with excess oxygen, 10 moles of nitrogen monoxide will be produced.
Example 2: Calculate the molar mass of ammonia (NH₃).
Solution: To calculate the molar mass of ammonia, we need to determine the atomic masses of nitrogen (N) and hydrogen (H) and add them up.
Atomic mass of nitrogen (N) = 14.01 g/mol
Atomic mass of hydrogen (H) = 1.01 g/mol
Since ammonia (NH₃) consists of one nitrogen atom and three hydrogen atoms, we multiply the atomic masses of nitrogen and hydrogen by their respective coefficients and add them up:
Molar mass of ammonia (NH₃) = (1 × atomic mass of nitrogen) + (3 × atomic mass of hydrogen)
= (1 × 14.01 g/mol) + (3 × 1.01 g/mol)
= 14.01 g/mol + 3.03 g/mol
= 17.04 g/mol
Therefore, the molar mass of ammonia (NH₃) is 17.04 g/mol.
Example 3: Determine the number of moles in 25 grams of ammonia (NH₃).
Solution: To find the number of moles in a given mass of ammonia, we need to use the molar mass of ammonia.
Molar mass of ammonia (NH₃) = 17.04 g/mol
Number of moles = Mass of substance / Molar mass
= 25 g / 17.04 g/mol
≈ 1.47 moles
Therefore, there are approximately 1.47 moles of ammonia (NH₃) in 25 grams.
Frequently Asked Question on Ammonia Formula
What is the chemical formula of ammonia?
The chemical formula of ammonia is NH3.
What elements are present in the formula of ammonia?
The formula of ammonia consists of one nitrogen (N) atom and three hydrogen (H) atoms.
What is the molecular shape of ammonia?
Ammonia has a trigonal pyramidal molecular shape, where the nitrogen atom occupies the central position, and the three hydrogen atoms are arranged symmetrically around it.
Is ammonia an organic or inorganic compound?
Ammonia is considered an inorganic compound because it does not contain carbon hydrogen bonds
What is the molar mass of ammonia?
The molar mass of ammonia NH3 is approximately 17.03 grams per mole g/mol.
What is the odour of ammonia?
Ammonia has a pungent, sharp odour often described as similar to that of household cleaning products or urine.
What are the physical states of ammonia?
At room temperature and standard pressure, ammonia is a colourless gas. However, under certain conditions, it can be condensed into a liquid or solid form.
What is the solubility of ammonia in water?
Ammonia is highly soluble in water, forming a solution called ammonium hydroxide. This solution is alkaline in nature.
What are the common uses of ammonia?
Ammonia has various applications, including its use in fertilizers, cleaning agents, refrigerants, and the production of various chemicals and materials.
Is ammonia toxic or hazardous?
Ammonia can be toxic and potentially hazardous, especially in high concentrations. It can cause respiratory irritation and damage. Proper safety precautions should be taken when handling and using ammonia.








