Table of Contents
Barium iodide is a chemical compound represented by the formula BaI2. It is a crystalline solid consisting of barium cations (Ba2+) and iodide anions (I–). Barium iodide is soluble in water and can be used in various applications, including scintillation counters, scintillator lamps, chemical synthesis, electroplating, and X-ray imaging. It plays a role in radiation detection, as a source of barium and iodide ions, and as a contrast agent in medical imaging. However, it’s important to handle barium iodide with caution as it can be toxic.
Formula of Barium Iodide
The chemical formula of Barium iodide is BaI2.
Barium iodide has the chemical formula BaI2. This compound is composed of one barium ion (Ba2+) and two iodide ions (I-). Barium, as a group 2 alkaline earth metal, has a 2+ charge, while iodide, as a halide ion, carries a single negative charge.
In barium iodide, the barium ion, with a +2 charge, combines with two iodide ions, each with a -1 charge, to achieve overall charge neutrality. The ionic bonding between the barium and iodide ions results in the formation of a crystal lattice structure.
The formula BaI2 reflects the stoichiometry of the compound, indicating that there are two iodide ions for every barium ion. This balanced combination ensures that the compound has a neutral charge overall.
Barium iodide is typically a white or colorless crystalline solid and is commonly used in various applications, including as a reagent in organic synthesis and in some medical procedures like radiographic imaging.
Structure of Barium Iodide
Barium iodide consists of a barium cation (Ba2+) and two iodide anions (I–) held together by ionic bonds. The structure can be represented as BaI2.
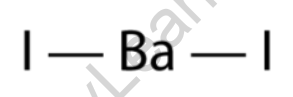
Physical Properties of Barium Iodide
– Appearance: Barium iodide is a white crystalline solid.
– Melting Point: It has a high melting point of around 856°C.
– Solubility: Barium iodide is soluble in water and forms a colorless solution.
Chemical Properties of Barium Iodide
– Reactivity: Barium iodide is a source of iodide ions and can undergo various chemical reactions. It can react with other substances to form new compounds.
– Hygroscopic: Barium iodide is hygroscopic, meaning it readily absorbs moisture from the air.
– Oxidation: Barium iodide can act as an oxidizing agent in certain reactions, facilitating the transfer of electrons.
Uses of Barium Iodide
– Chemical Reagent: Barium iodide is used as a chemical reagent in laboratories for various reactions and synthesis processes.
– X-ray Imaging: Barium iodide is used in radiography and X-ray imaging as a contrast agent. It helps enhance the visibility of certain body parts or organs during medical procedures.
– Scintillation Counters: Barium iodide is employed in scintillation counters, which are devices used to detect and measure ionizing radiation.
– Manufacture of Barium Compounds: Barium iodide is used as a precursor or starting material for the production of other barium compounds, such as barium sulfate or barium carbonate.
It’s important to handle barium iodide with care as it is toxic and can cause health hazards. Proper safety precautions should be followed when working with this compound.
Solved questions on the formula of Barium Iodide (BaI2):
Example 1: What is the molar mass of Barium Iodide?
Solution:
The molar mass of Barium iodide can be calculated by summing the atomic masses of its constituent elements. The atomic mass of Barium (Ba) is 137.33 g/mol, and the atomic mass of iodine (I) is 126.90 g/mol. Since there are two iodine atoms in the formula, we multiply the atomic mass of iodine by 2.
Molar mass of BaI2 = Atomic mass of Ba + (Atomic mass of I × 2)
= 137.33 g/mol + (126.90 g/mol × 2)
= 137.33 g/mol + 253.80 g/mol
= 391.13 g/mol
Therefore, the molar mass of Barium iodide (BaI2) is 391.13 g/mol.
Example 2: How many moles of iodine are present in 5 grams of Barium iodide?
Solution:
To determine the number of moles of iodine in 5 grams of Barium iodide, we need to use the molar mass of BaI2 and the given mass.
Molar mass of BaI2 = 391.13 g/mol
Number of moles = Given mass / Molar mass
= 5 g / 391.13 g/mol
≈ 0.0128 mol (rounded to four decimal places)
Therefore, there are approximately 0.0128 moles of iodine in 5 grams of Barium iodide.
Frequently Asked Questions on Barium Iodide
Is barium iodide soluble or insoluble?
Barium iodide (BaI2) is soluble in water. When BaI2 is dissolved in water, it dissociates into barium cations (Ba2+) and iodide anions (I-). The solubility of BaI2 is relatively high, meaning a significant amount of it can dissolve in water to form a clear solution. However, it's worth noting that the solubility of BaI2 may decrease as the temperature decreases. Additionally, like other barium compounds, barium iodide can be toxic and should be handled with caution.
What is barium iodine used for?
Barium iodide is primarily used in scintillation counters and scintillator lamps for radiation detection and measurement. It also finds application as a source of barium and iodide ions in chemical synthesis, as an electrolyte in electroplating, and as a contrast agent in X-ray imaging.
Why is barium iodide soluble?
Barium iodide is soluble because it undergoes ionization in water, breaking down into barium cations (Ba2+) and iodide anions (I-). These ions are surrounded by water molecules, which stabilize them and allow them to remain in solution. The interaction between the ions and water molecules, specifically through ion-dipole interactions, helps overcome the attractive forces within the solid and allows for the dissolution of barium iodide in water.
What holds a sample of barium iodide together?
A sample of barium iodide is held together by ionic bonds. In the solid state, the barium cations (Ba2+) and iodide anions (I-) are attracted to each other through electrostatic forces, forming a lattice structure. These strong ionic bonds between the positively charged barium ions and negatively charged iodide ions are responsible for holding the sample of barium iodide together.
What happens when barium iodide and potassium bromide react with each other?
When barium iodide (BaI2) and potassium bromide (KBr) react with each other, a double displacement reaction occurs. The barium cations (Ba2+) from barium iodide and the bromide anions (Br-) from potassium bromide switch places to form barium bromide (BaBr2) and potassium iodide (KI). The balanced chemical equation for this reaction is: BaI2 + 2KBr → BaBr2 + 2KI
What is the Colour of barium iodide solution?
The color of a barium iodide solution can vary depending on the concentration. Dilute solutions of barium iodide are typically colorless or slightly yellowish. However, as the concentration increases, the solution may appear yellow or even amber in color.
What bond is barium iodide?
Barium iodide (BaI2) forms an ionic bond. An ionic bond is formed between the barium cation (Ba2+) and the iodide anion (I-). In this type of bond, the electrons are transferred from the metal (barium) to the nonmetal (iodine) to achieve charge balance and form a stable compound. The ionic bond in barium iodide is characterized by the strong electrostatic attraction between the positive and negative ions, resulting in the formation of a solid crystal lattice structure.




