Table of Contents
Introduction to Chromate Formula
Chromate refers to a chemical species or an ion that contains one central chromium atom (Cr) bonded to four oxygen atoms (O) in a tetrahedral arrangement. The chemical formula for chromate is CrO42-. The chromate ion is commonly found in various compounds and salts, such as potassium chromate (K2CrO4) and lead chromate (PbCrO4).
Chromates have distinctive yellow coloration and are often used as pigments in dyes and paints. They are also important in various industrial applications, including electroplating, corrosion inhibition, and as catalysts in chemical reactions. Additionally, chromates are utilized in analytical chemistry as indicators and reagents for specific reactions.
Formula of Chromate
The formula for the chromate ion is CrO42-. In this formula, Cr represents the central chromium atom, and O represents oxygen. The superscript 2- indicates that the chromate ion carries a charge of -2, indicating a net negative charge.
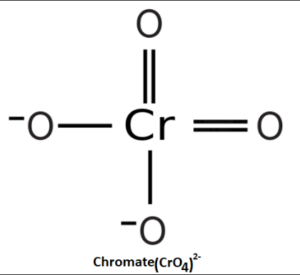
Structure of Chromate
The chromate ion (CrO42-) has a tetrahedral structure, where the central chromium atom is surrounded by four oxygen atoms. Each oxygen atom is covalently bonded to the chromium atom, forming four Cr-O bonds. The bond lengths and angles in the chromate ion are determined by the electron arrangement around the central chromium atom.
The tetrahedral arrangement of the chromate ion results in a symmetric distribution of charge, with the negative charge spread evenly across the oxygen atoms. The chromate ion is stabilized by the electrostatic attraction between the negatively charged oxygen atoms and the positively charged chromium ion.
It’s important to note that chromate compounds can exist in different oxidation states, such as hexavalent chromium (Cr6+) in chromate, as well as other forms like dichromate (Cr2O72-) and trichromate (Cr3O102-). These different forms have distinct structures and properties but all share the fundamental arrangement of chromium and oxygen atoms in the chromate ion.
Physical Properties of Chromate
– Color: Chromate compounds are typically yellow in color, with variations depending on the specific compound.
– Solubility: Chromates are generally soluble in water. The solubility can vary depending on the specific chromate compound and the conditions such as pH and temperature.
– Melting and Boiling Points: The melting and boiling points of chromate compounds vary depending on the specific compound. For example, lead chromate has a melting point of 844°C (1551°F).
– Crystal Structure: Chromate compounds often form crystalline structures, with different crystal systems depending on the specific compound.
Chemical Properties of Chromate
– Oxidizing Agent: Chromates are strong oxidizing agents. They have the ability to accept electrons from other substances in redox reactions, leading to their reduction. This property is especially prominent in hexavalent chromium compounds (Cr6+).
– Acid-Base Reactions: Chromates can act as both acids and bases. In acidic conditions, chromates can form dichromate ions (Cr2O72-). In basic conditions, they can undergo hydrolysis reactions, producing chromite ions (CrO42-) and hydroxide ions (OH–).
– Reactivity with Reducing Agents: Chromates can react with reducing agents, such as organic compounds or metals, by accepting electrons and undergoing reduction reactions. This reduction can result in the formation of lower oxidation state chromium compounds.
– Stability: The stability of chromate compounds varies depending on the specific compound and conditions. For example, some chromates are stable under certain pH conditions, while others may undergo hydrolysis or decomposition reactions.
Uses of Chromate
– Chromates have various applications, primarily due to their oxidizing properties and yellow color.
– In industrial settings, chromates are used to produce pigments, such as lead chromate (PbCrO4), a yellow pigment commonly known as chrome yellow.
– Chromate compounds are also utilized as corrosion inhibitors in coatings, especially for metals like zinc and aluminum.
– Additionally, chromates have been used in some electroplating processes and in tanning leather.
Conclusion
In conclusion, the chromate ion, with the chemical formula CrO42-, consists of one chromium atom (Cr) bonded to four oxygen atoms (O). It is found in compounds and salts, and it is characterized by its yellow coloration. Chromates are widely used in industries such as dyeing, painting, electroplating, and chemical catalysis. They also find applications in analytical chemistry as indicators and reagents. The unique properties and versatility of chromates make them important compounds in various fields.
It’s important to note that some chromate compounds, such as hexavalent chromium compounds, have been associated with environmental and health concerns. Hexavalent chromium is toxic and carcinogenic, so handling and disposing of chromate compounds properly and following safety guidelines when working with them is crucial.
Solved examples on the Chromate formula
Example 1: What is the formula for a compound formed when potassium reacts with chromate?
Solution:
– The potassium cation has a charge of +1, while the chromate anion has a charge of -2.
– To balance the charges, we need two potassium ions for every chromate ion.
– Therefore, the formula for the compound formed is K2CrO4.
Example 2: How many oxygen atoms are present in one chromate ion?
Solution:
– The formula for the chromate ion is CrO42-.
– In one chromate ion, there are four oxygen atoms (O).
– Therefore, there are a total of four oxygen atoms in one chromate ion.
Frequently Asked Questions on Chromate Formula
What is the common use of chromate?
One common use of chromate compounds is as pigments. It is mainly used as an inhibitor of corrosion, as a primer, as a decorative finish, or to maintain electrical conductance. Specifically, lead chromate (PbCrO4) is widely used as a yellow pigment in various applications. Some common uses include: - Paints and Coatings: Lead chromate is used as a pigment in paints and coatings, providing a bright yellow color. It is often used in automotive coatings, architectural paints, and industrial coatings. - Plastics and Polymers: Lead chromate is also incorporated into plastics and polymers to achieve a vibrant yellow color. It is used in the manufacturing of plastic products, such as toys, packaging materials, and consumer goods. Additionally, chromate compounds have been utilized in other applications in the past, such as in the production of dyes, inks, and ceramics. However, due to concerns over the toxicity of hexavalent chromium (Cr6+), the use of certain chromate compounds has been restricted or replaced with safer alternatives in many industries. It is important to note that the use of chromate compounds should adhere to local regulations and safety guidelines to minimize environmental and health risks.
What is the common name of chromate?
The common name of chromate is simply chromate. Chromate compounds are commonly referred to by their specific names, such as lead chromate, potassium chromate, or zinc chromate, depending on the specific metal cation associated with the chromate ion. Another name for chromate is bichromate. This term is used specifically for the dichromate ion (Cr2O72-), which is derived from the chromate ion (CrO42-). The dichromate ion is an oxidizing agent and has a reddish-orange color. It is commonly referred to as bichromate because it contains two chromium atoms (di-) in its structure. It is also called chromium oxoanion or divalent inorganic anions.
Is chromate acidic or basic?
The chromate ion is the predominant species in alkaline solutions, but in acidic solutions, dichromate can become the predominant ion.
Is chromate polar or nonpolar?
The chromate ion (CrO42-) is a polyatomic ion, and its polarity can be determined by considering the individual bond polarities and the overall molecular geometry. In the chromate ion, each oxygen atom is covalently bonded to the central chromium atom. Oxygen is more electronegative than chromium, so the oxygen-chromium bonds are polar, with oxygen having a partial negative charge (δ-) and chromium having a partial positive charge (δ+). When considering the overall molecular geometry of the chromate ion, it adopts a tetrahedral arrangement with the central chromium atom at the center and the four oxygen atoms surrounding it. The bond dipoles of the oxygen-chromium bonds cancel each other out symmetrically due to the tetrahedral geometry. As a result, the chromate ion is considered to be nonpolar. However, it's important to note that when the chromate ion interacts with other polar molecules or ions, such as in solution or in chemical reactions, the overall polarity of the system can be influenced by those interactions.
What is potassium chromate used for?
It is used as a fungicide in chemical processing, for making pigments for paints and inks, and for producing other chromium compounds. Potassium chromate is a potassium salt in a ratio of 2:1 consisting of potassium and chromate ions. It has a role as both a carcinogenic and an oxidizing agent.








