Table of Contents
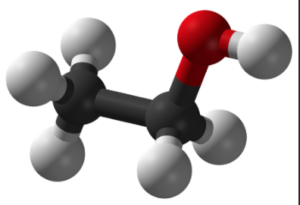
Ethanol is a versatile and widely used organic compound that is commonly known as alcohol. Its chemical formula is C2H5OH, which represents its composition of two carbon (C) atoms, six hydrogen (H) atoms, and one oxygen (O) atom.
Ethanol exhibits a range of physical and chemical properties that make it valuable for various applications. Here are some key properties:
Physical Properties of Ethanol
- State: Ethanol is a colorless liquid at room temperature and standard pressure.
- Odor: It has a characteristic sweet, fruity odor.
- Density: The density of ethanol is approximately 0.789 g/mL, making it less dense than water.
- Boiling Point: Ethanol has a relatively low boiling point of 78.4 degrees Celsius (173.2 degrees Fahrenheit), which allows it to evaporate easily.
- Melting Point: The melting point of ethanol is -114.1 degrees Celsius (-173.4 degrees Fahrenheit), resulting in a liquid state at typical ambient conditions.
- Solubility: Ethanol is highly soluble in water and most organic solvents. It forms homogeneous mixtures when combined with water in various proportions.
- Refractive Index: Ethanol has a refractive index of around 1.36, which influences its optical properties.
Chemical Properties of Ethanol
- Combustibility: Ethanol is highly flammable and burns in the presence of an ignition source, producing carbon dioxide and water as combustion products.
- Oxidation: Ethanol can undergo oxidation reactions to form ethanal (acetaldehyde) and further oxidize to ethanoic acid (acetic acid). These reactions are commonly used in organic chemistry and biochemistry.
- Acid-Base Properties: Ethanol can act as a weak acid or a weak base. It can donate a proton (H+) to acceptors or accept a proton to form ethoxide ions (C2H5O–).
- Reaction with Oxygen: Ethanol can react with molecular oxygen (O2) in the presence of certain catalysts to form ethanal (acetaldehyde) or ethanoic acid (acetic acid).
- Esterification: Ethanol can undergo esterification reactions with organic acids, producing esters and water. These reactions are commonly used in the production of fragrances and flavors.
- Solvent Properties: Ethanol is a versatile solvent that can dissolve a wide range of organic and inorganic compounds. This property contributes to its use in the pharmaceutical, cosmetic, and cleaning industries.
Ethanol finds widespread use in various applications, including as a fuel, solvent, disinfectant, beverage, and precursor for the synthesis of numerous chemicals. Understanding its formula and properties is crucial for its safe handling, industrial processes, and its impact on human health.
Structure of Ethanol Formula
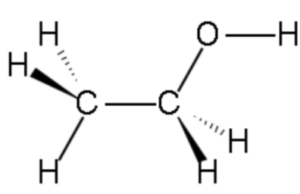
- Ethanol, with the chemical formula C2H5OH, is a simple organic compound belonging to the alcohol functional group. Its structure consists of two carbon atoms (C2), six hydrogen atoms (H6), and one oxygen atom (O).
- In the structure, the two carbon atoms are bonded together by a single covalent bond, forming a carbon-carbon (C-C) bond. One of the carbon atoms is also bonded to three hydrogen atoms, while the other carbon atom is bonded to one hydrogen atom and the oxygen atom.
- The oxygen atom is bonded to the carbon atom through a single covalent bond, forming a carbon-oxygen (C-O) bond. This bond gives ethanol its characteristic properties as an alcohol.
- The structure of ethanol reveals that it has a polar nature due to the presence of the oxygen atom. Oxygen is more electronegative than carbon or hydrogen, resulting in an uneven distribution of electron density. As a result, the oxygen atom carries a partial negative charge (δ-) while the hydrogen atoms carry partial positive charges (δ+).
- The presence of the hydroxyl group (-OH) in the structure is characteristic of alcohols. This hydroxyl group imparts various properties to ethanol, including its ability to form hydrogen bonds with other molecules, influence its solubility in water, and contribute to its reactivity in chemical reactions.
- Understanding the structure of ethanol is essential for comprehending its physical and chemical properties, as well as its behavior in various applications. It provides the foundation for studying the reactions and interactions of ethanol with other compounds, as well as its role in biological processes and industrial applications.
Solved Examples on Ethanol Formula
Example 1: How many moles of ethanol are present in 250 grams of ethanol (C2H5OH)?
Solution: To determine the number of moles of ethanol, we need to use the molar mass of ethanol.
The molar mass of ethanol (C2H5OH) can be calculated by adding up the atomic masses of its constituent elements: C (carbon) = 12.01 g/mol H (hydrogen) = 1.008 g/mol O (oxygen) = 16.00 g/mol
Molar mass of ethanol (C2H5OH) = (2 * C) + (6 * H) + O = (2 * 12.01) + (6 * 1.008) + 16.00 = 46.07 g/mol
Given that the mass of ethanol is 250 grams, we can use the molar mass to calculate the number of moles:
Number of moles = Mass / Molar mass = 250 g / 46.07 g/mol ≈ 5.43 moles
Therefore, there are approximately 5.43 moles of ethanol in 250 grams of ethanol.
Example 2: Calculate the percent composition of carbon (C), hydrogen (H), and oxygen (O) in ethanol (C2H5OH).
Solution: To calculate the percent composition, we need to determine the molar mass of each element and the total molar mass of ethanol.
Molar mass of carbon (C) = 12.01 g/mol
Molar mass of hydrogen (H) = 1.01 g/mol
Molar mass of oxygen (O) = 16.00 g/mol
Molar mass of ethanol (C2H5OH) = (2 × molar mass of carbon) + (6 × molar mass of hydrogen) + (1 × molar mass of oxygen)
= (2 × 12.01 g/mol) + (6 × 1.01 g/mol) + (1 × 16.00 g/mol)
= 24.02 g/mol + 6.06 g/mol + 16.00 g/mol
= 46.08 g/mol
Now, we can calculate the percent composition of each element:
Percent composition of carbon (C) = (2 × molar mass of carbon) / molar mass of ethanol × 100%
= (2 × 12.01 g/mol) / 46.08 g/mol × 100%
≈ 52.05%
Percent composition of hydrogen (H) = (6 × molar mass of hydrogen) / molar mass of ethanol × 100%
= (6 × 1.01 g/mol) / 46.08 g/mol × 100%
≈ 13.10%
Percent composition of oxygen (O) = (1 × molar mass of oxygen) / molar mass of ethanol × 100%
= (1 × 16.00 g/mol) / 46.08 g/mol × 100%
≈ 34.85%
Therefore, the percent composition of carbon, hydrogen, and oxygen in ethanol (C₂H₅OH) is approximately 52.05%, 13.10%, and 34.85%, respectively.
Example 3: Determine the number of moles in 100 grams of ethanol (C2H5OH).
Solution: To find the number of moles in a given mass of ethanol, we need to use the molar mass of ethanol.
Molar mass of ethanol (C2H5OH) = 46.08 g/mol (as calculated in Example 1)
Number of moles = Mass of substance / Molar mass
= 100 g / 46.08 g/mol
≈ 2.17 moles
Therefore, there are approximately 2.17 moles of ethanol (C₂H₅OH) in 100 grams.
Frequently Asked Question on Ethanol
What is the chemical formula of ethanol?
The chemical formula of ethanol is C2H5OH.
What are the elements present in the formula of ethanol?
The formula of ethanol consists of two carbon C atoms, six hydrogen H atoms, and one oxygen O atom.
Is ethanol the same as ethyl alcohol?
Yes, ethanol is commonly referred to as ethyl alcohol. It is the primary alcohol found in alcoholic beverages.
What is the structural formula of ethanol?
The structural formula of ethanol can be represented as CH3CH2OH, indicating the arrangement of atoms and bonds in the molecule.
What is the molar mass of ethanol?
The molar mass of ethanol is approximately 46.07 grams per mole g/mol.
Is ethanol a polar molecule?
Yes, ethanol is a polar molecule due to the presence of the polar C-O bond and the uneven distribution of electron density caused by the oxygen atom.
What is the boiling point of ethanol?
The boiling point of ethanol is approximately 78.4 degrees Celsius 173.2 degrees Fahrenheit.
Is ethanol soluble in water?
Yes, ethanol is highly soluble in water due to its ability to form hydrogen bonds with water molecules. This property makes it miscible in all proportions with water.
Is ethanol flammable?
Yes, ethanol is highly flammable and can ignite easily. It has a low flash point and can be used as a fuel or as a solvent in flammable liquid mixtures.
What are the common uses of ethanol?
Ethanol has numerous applications, including as a fuel additive, solvent in industries, disinfectant, the ingredient in alcoholic beverages, and precursor for the synthesis of various chemicals.






