Iron Oxide Formula
Introduction
Iron oxide refers to a group of chemical compounds composed of iron and oxygen. It is commonly found in nature and has several different forms, including FeO (iron(II) oxide), Fe2O3 (iron(III) oxide), and Fe3O4 (iron(II,III) oxide), also known as magnetite.
Iron oxide compounds are known for their characteristic colours, which range from black to reddish-brown, depending on the specific form and the presence of impurities.
For example, FeO is black, Fe2O3 is red, and Fe3O4 is black or dark brown.
Uses of Iron Oxide
Iron oxide compounds have various applications across different industries. They are commonly used as pigments in paints, coatings, and ceramics due to their vibrant colours. Iron oxide nanoparticles have also gained attention in medical and environmental fields for their potential uses in drug delivery systems, magnetic resonance imaging (MRI), and wastewater treatment.
In addition to their practical applications, iron oxide compounds also play a role in geological processes. They can be found in rocks, soil, and minerals, and their presence can provide insights into the history and composition of geological formations.
Overall, iron oxide compounds are important substances with diverse uses and can be found in various natural and synthetic forms.
Structural Formula of Iron Oxide
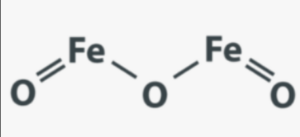
Iron oxide refers to a group of compounds that can have different structural formulas depending on the specific form of iron oxide.
Physical Properties of Iron Oxide
- Colour: Iron oxide can have various colours, including red, brown, black, and yellow, depending on the specific form. For example, iron(III) oxide (Fe2O3) is commonly known as rust and appears as a reddish-brown colour.
- Density: The density of iron oxide varies depending on the specific form and can range from 3.4 to 5.2 grams per cubic centimeter (g/cm3).
- Melting and boiling points: The melting and boiling points of iron oxide depend on the specific form. For example, iron(III) oxide (Fe2O3) has a melting point of approximately 1,565 degrees Celsius (2,849 degrees Fahrenheit).
- Solubility: Iron oxide is generally insoluble in water. However, it can react with acids and form soluble iron salts.
- Magnetism: Some forms of iron oxide, such as magnetite (Fe3O4), exhibit magnetic properties and can be attracted to a magnet.
- Hardness: Iron oxide is relatively hard, with a Mohs hardness of 5.5 to 6.5, depending on the specific form.
Chemical Properties of Iron Oxide Formula
- Reactivity with acids: Iron oxide can react with acids to form iron salts and water. For example, when iron(III) oxide reacts with hydrochloric acid, it forms iron(III) chloride and water.
Fe2O3 + 6HCl → 2 FeCl3 + 3H2O
- Redox reactions: Iron oxide can participate in redox reactions, where it can either act as an oxidizing agent or a reducing agent depending on the reaction conditions. For example, iron(III) oxide can be reduced to metallic iron when reacted with carbon monoxide.
Fe2O3 + 3CO → 2Fe + 3CO2
- Catalytic properties: Iron oxide can exhibit catalytic activity in various chemical reactions. It is commonly used as a catalyst in industrial processes such as the Haber-Bosch process for ammonia synthesis.
- Stability: Iron oxide is relatively stable under normal conditions and does not readily decompose or react with air or moisture. However, prolonged exposure to moisture and oxygen can lead to the formation of rust, which is a hydrated form of iron(III) oxide.
- Photocatalytic properties: Certain forms of iron oxide, such as hematite (α-Fe2O3), exhibit photocatalytic activity. They can absorb light energy and participate in photochemical reactions, such as the degradation of organic pollutants in the presence of light.
Solved Examples on Iron Oxide Formula
Example 1: Iron(III) oxide reacts with carbon monoxide to produce iron and carbon dioxide. Write the balanced chemical equation for this reaction.
Solution: Fe2O3 + 3CO → 2Fe + 3CO2
Example 2: Iron(II) oxide is heated in the presence of oxygen gas to form iron(III) oxide. Write the balanced chemical equation for this reaction.
Solution: 4FeO + O2 → 2Fe2O3
Example 3: Iron(III) oxide reacts with sulfuric acid to form iron(III) sulfate and water. Write the balanced chemical equation for this reaction.
Solution: Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O
Conclusion
In conclusion, iron oxide is a compound composed of iron and oxygen. It exists in various forms, including rust (Fe2O3), which is a common example of iron oxide. Iron oxide has several important properties and applications. It is known for its characteristic reddish-brown color and is often used as a pigment in paints, coatings, and ceramics. It is also used in the manufacturing of magnetic materials, such as magnetic tapes and hard drives. Iron oxide has been utilized in the field of medicine as a contrast agent in imaging studies, such as MRI scans. Additionally, iron oxide nanoparticles have gained attention for their potential applications in various fields, including drug delivery, environmental remediation, and catalysis. Overall, iron oxide plays a significant role in both industrial and scientific sectors.
Frequently Asked Questions on Iron oxide Formula
1: What are the possible formulas for iron oxide?
Answer: There are multiple possible formulas for iron oxide, depending on the oxidation state of iron and the ratio of iron to oxygen. The most common formulas for iron oxide include:
- Iron(II) oxide: FeO
- Iron(III) oxide: Fe2O3
- Iron(II,III) oxide (also known as magnetite): Fe3O4
These formulas represent different stoichiometries and oxidation states of iron in the oxide compound. Iron(II) oxide contains iron in the +2 oxidation state, while iron(III) oxide contains iron in the +3 oxidation state. Iron(II,III) oxide, or magnetite, contains a combination of iron in both +2 and +3 oxidation states.
2: What is the most common form of iron oxide?
Answer: The most common form of iron oxide is iron(III) oxide, also known as ferric oxide or rust. Its chemical formula is Fe2O3. Iron(III) oxide occurs naturally as the mineral hematite and is responsible for the reddish-brown colouration of rusted iron. It is widely found in nature and is an important iron ore.
3: Is Iron oxide acidic or basic?
Answer: Iron(III) oxide (Fe2O3) is considered to be amphoteric, which means it can exhibit both acidic and basic properties depending on the reaction conditions. In aqueous solutions, Fe2O3 can react with acids to form iron(III) salts, indicating its acidic nature. On the other hand, Fe2O3 can also react with strong bases to form iron(III) hydroxide, demonstrating its basic nature. The amphoteric behavior of Fe2O3 allows it to participate in various chemical reactions, making it a versatile compound.
4: What are 3 facts about iron oxide?
Answer:
- Common Occurrence: Iron oxide is one of the most abundant minerals on Earth and is found in various forms, including hematite (Fe2O3) and magnetite (Fe3O4). It is commonly found in rocks, soils, and sedimentary deposits.
- Red Colour: Iron oxide is known for its characteristic red colour, which gives it the common name “rust.” This red colour is seen in the iron(III) oxide (Fe2O3) form and is often associated with corrosion and oxidation of iron or steel surfaces.
- Industrial Applications: Iron oxide has numerous industrial applications. It is used as a pigment in paints, coatings, and ceramics due to its stable colour and resistance to fading. Iron oxide nanoparticles are used in various fields, including medicine, electronics, and environmental remediation, due to their unique properties and reactivity.
5: What is iron oxide also known as?
Iron oxide, also called ferric oxide is an inorganic compound with the chemical formula Fe2O3.
6: Is iron oxide Fe2O3 or Fe3O4?
Answer: Iron oxide can refer to different compounds with varying chemical formulas. The two most common forms of iron oxide are Fe2O3 (iron(III) oxide) and Fe3O4 (iron(II,III) oxide), also known as magnetite.
Fe2O3: Iron(III) oxide, commonly known as rust, is a reddish-brown compound. It is formed when iron reacts with oxygen in the presence of moisture. Fe2O3 is the main component of red iron oxide pigments used in paints, coatings, and ceramics. It is also found in natural mineral forms, such as hematite.
Fe3O4: Iron(II,III) oxide, or magnetite, is a black or dark grey compound. It is a naturally occurring mineral and is one of the main ores of iron. Fe3O4 is magnetic and has unique magnetic properties, making it useful in various applications, including magnetic storage media, catalysts, and as a magnet in scientific experiments.
So, both Fe2O3 and Fe3O4 can be referred to as iron oxide, but they have different chemical compositions and properties.
7: Are iron oxides used in cosmetics?
Answer: Iron oxides are commonly used in cosmetics due to their natural colors and safe properties. They are widely used as pigments to provide color to various cosmetic products, including makeup, skincare, and hair care products. The three primary colors of iron oxide pigments used in cosmetics are red (Fe2O3), yellow (Fe2O3•H2O), and black (Fe3O4).
In cosmetics, iron oxides are preferred over other colorants because they are well-tolerated by the skin and have low allergenic potential. They provide a wide range of shades and can be blended to create different hues. Iron oxide pigments are used in foundations, blushes, eyeshadows, lipsticks, nail polishes, and other cosmetic products to add color, enhance pigmentation, and create desired effects.
Furthermore, iron oxides are known for their excellent light stability, which means they do not fade or change color when exposed to sunlight or other environmental factors. This makes them suitable for long-lasting cosmetic formulations.
It’s worth noting that iron oxides used in cosmetics are typically synthetic or refined mineral pigments, ensuring their purity and safety for use on the skin. Manufacturers adhere to strict quality standards and regulatory guidelines to ensure the safety and effectiveness of cosmetic products containing iron oxides.
8: What is the colour of Iron oxide?
Answer: Iron oxide exhibits a range of colors depending on its chemical composition and crystal structure. The most common colors of iron oxide are:
- Red: Iron(III) oxide (Fe2O3) is commonly known as red iron oxide or rust. It imparts a deep red or reddish-brown color and is often used as a pigment in paints, coatings, ceramics, and cosmetics.
- Yellow: Iron(III) oxide-hydroxide (FeO(OH)) is a hydrated form of iron oxide that appears yellow in color. It is used as a yellow pigment in various applications.
- Brown: A mixture of iron(III) oxide and iron(II) oxide (Fe3O4) is known as brown iron oxide. It exhibits a dark brown to blackish-brown color and is used as a pigment in paints, coatings, and pigmented concrete.
- Black: Iron(II,III) oxide (Fe3O4), also known as magnetite, is a black-colored iron oxide. It is commonly used in magnetic recording media, magnetic nanoparticles, and as a black pigment in various applications.




