Table of Contents
Lead Acetate Formula
Lead acetate, with the chemical formula Pb(CH3COO)2, is a compound consisting of lead (Pb) cations and acetate (CH3COO–) anions. It is also known as lead(II) acetate or sugar of lead. Lead acetate is a white crystalline solid that is soluble in water.
It is commonly used in various industrial applications, such as in the production of dyes, pigments, and hair dyes. It is also used in some laboratory procedures and as a reagent in certain chemical reactions. However, lead acetate is toxic and must be handled with care.
Structural Formula of Lead Acetate
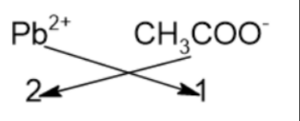
It is an ionic compound that is formed by the reaction of elemental lead and acetic acid. It has one Pb2+ ion and two CH3COO– ions. As lead cation contains +2 charge while each acetate anion has -1 charge, so the compound lead(II) acetate contains zero charges.
Uses of Lead Acetate
- Hair Dye: Lead acetate is used in some hair dyes to darken hair. It reacts with sulfur-containing amino acids in the hair, forming dark-colored lead sulfide compounds.
- Analytical Chemistry: Lead acetate is used in analytical chemistry as a reagent for the detection and determination of hydrogen sulfide (H2S) in gas and liquid samples.
- Pigments: Lead acetate has been historically used as a pigment in ceramics, paints, and cosmetics. However, due to concerns about its toxicity, its use in these applications has significantly decreased.
- Laboratory Reagent: Lead acetate is used as a laboratory reagent for various purposes, such as precipitation reactions, organic synthesis, and as a starting material for the preparation of other lead compounds.
Physical Properties of Lead Acetate
- Appearance: Lead acetate typically appears as a white crystalline solid or a fine white powder.
- Odour: It is odorless.
- Solubility: Lead acetate is soluble in water and forms a clear solution. It is also soluble in alcohol and glycerol.
- Density: The density of lead acetate is approximately 3.25 g/cm³.
- Melting Point: Lead acetate has a melting point of around 280-300°C (536-572°F).
- Boiling Point: When heated, lead acetate decomposes before boiling.
- Stability: Lead acetate is relatively stable under normal conditions. However, it can decompose when exposed to heat or strong acids, releasing toxic fumes.
- Toxicity: Lead acetate is highly toxic and should be handled with care. It can be absorbed through the skin, ingestion, or inhalation and can cause severe health effects.
Chemical Properties of Lead Acetate Formula
- Acidic Nature: Lead acetate is slightly acidic in nature due to the presence of acetate ions (CH3COO–) which can release hydrogen ions (H+) in aqueous solutions.
- Solubility: Lead acetate is highly soluble in water, and it readily dissociates into lead ions (Pb2+) and acetate ions (CH3COO–) when dissolved.
- Reaction with Acids: Lead acetate reacts with strong acids to form lead salts and acetic acid. For example, reacting lead acetate with sulfuric acid (H2SO4) yields lead sulfate (PbSO4) and acetic acid (CH3COOH). Pb(CH3COO)2 + H2SO4 → PbSO4 + 2CH3COOH
- Reaction with Alkalis: Lead acetate reacts with alkalis, such as sodium hydroxide (NaOH), to form lead hydroxide (Pb(OH)2) and sodium acetate (CH3COONa). Pb(CH3COO)2 + 2NaOH → Pb(OH)2 + 2CH3COONa
- Precipitation Reactions: Lead acetate can form precipitates with various anions, such as sulfate (SO42-), chloride (Cl–), and carbonate (CO32-), leading to the formation of insoluble lead salts.
- Redox Reactions: Lead acetate can participate in redox reactions, undergoing oxidation or reduction processes. For example, reacting lead acetate with hydrogen sulfide (H2S) gas results in the precipitation of lead sulfide (PbS) and the formation of acetic acid (CH3COOH). Pb(CH3COO)2 + H2S → PbS + 2CH3COOH
- Stability: Lead acetate is relatively stable under normal conditions. However, it can decompose at high temperatures, releasing toxic fumes and forming lead oxide (PbO).
Conclusion
In conclusion, lead acetate, with the chemical formula Pb(CH3COO)2, is a compound that finds applications in various fields. It is used in hair dyes, analytical chemistry for the detection of hydrogen sulfide, as a pigment in ceramics and paints (although its use has decreased due to toxicity concerns), and as a laboratory reagent for precipitation reactions and organic synthesis. However, it is important to emphasize that lead acetate is highly toxic and requires strict handling and disposal measures to prevent health risks and environmental contamination.
Frequently Asked Questions on Lead Acetate Formula
1: Why do we add lead acetate?
Answer: Lead acetate is sometimes added to certain chemical reactions or processes for specific purposes. Here are a few common reasons why lead acetate may be used:
- Detection of Hydrogen Sulphide: Lead acetate paper or solution is often used to detect the presence of hydrogen sulfide gas. When hydrogen sulfide reacts with lead acetate, it forms a black precipitate of lead sulfide, indicating the presence of the gas.
- Hair Colouring: Lead acetate has been historically used in some hair dyes and colorants. It reacts with sulfur-containing proteins in the hair to produce color changes. However, the use of lead acetate in cosmetic products is now restricted or prohibited in many countries due to its toxicity.
- Laboratory Reagent: Lead acetate can be used as a reagent in laboratory experiments and chemical reactions. It may be added for specific purposes such as precipitation reactions or the synthesis of other lead compounds.
2: What is the structure formula of lead acetate?
Answer: In this structural formula, the Pb represents the lead (Pb) atom, and the C, H, and O represent carbon, hydrogen, and oxygen atoms, respectively. The lines and bonds between the atoms indicate the connections and arrangements of the atoms in the molecule.
3: What is another name for lead acetate?
Answer: Lead(II) acetate, also known as lead acetate, lead diacetate, plumbous acetate, sugar of lead, lead sugar, salt of Saturn, or Goulard’s powder, is a white crystalline chemical compound with a slightly sweet taste.
4: What is the Valency of lead acetate?
Answer: The valency of lead radical ( Pb2+) is two and acetate compound radical ( CH3COO – ) is one. After criss-crossing the valency numbers, the formula can be written as PbCH3COO2 .
5: Why is lead acetate called sugar of lead?
Answer: Lead acetate is known in many names such as plumbous acetate, lead acetate, salt of Saturn, lead sugar or sugar of the lead etc. It is white crystalline in nature and because of its slightly sweet taste , it is commonly called as the lead sugar.
6: What is the structure of the glycerol?
Answer: The structure of glycerol, also known as glycerine or glycerin, is as follows:
HO-CH2-CH(OH)-CH2-OH
It consists of a three-carbon backbone with three hydroxyl (-OH) groups attached to each carbon atom. The hydroxyl groups give glycerol its characteristic properties and make it a versatile compound used in various applications.
7: What is another name for glycerol?
Answer: Another name for glycerol is glycerin.
8: Is glycerol a lipid or protein?
Answer: Glycerol is a type of molecule known as a sugar alcohol. It is neither a lipid nor a protein. Lipids are a diverse group of molecules that include fats, oils, and certain types of steroids, while proteins are large, complex molecules composed of amino acids. Glycerol, on the other hand, is a small, three-carbon molecule that is often a component of lipids called triglycerides.








