Magnesium Phosphate Formula
Introduction
Magnesium phosphate is a chemical compound with the formula Mg3(PO4)2. It is composed of three magnesium ions (Mg2+) and two phosphate ions (PO43-). Magnesium phosphate exists in several forms, including anhydrous (without water) and hydrated (with water) compounds. It is commonly used in various applications such as fertilizers, food additives, and in the production of pharmaceuticals.
Structural Formula of Magnesium Phosphate
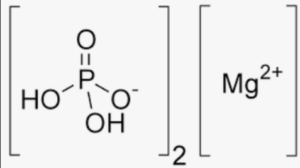
The structural formula of magnesium phosphate can vary depending on the specific form or stoichiometry. One common form is magnesium phosphate trihydrate, which has the formula Mg3(PO4)2·3H2O. In this compound, three magnesium ions (Mg2+) are bonded to two phosphate ions (PO43–), and three water molecules (H2O) are also present in the structure.
Uses of Magnesium Phosphate
- Fertilizer: Magnesium phosphate is used as a source of magnesium and phosphorus in fertilizers. It provides essential nutrients for plant growth and helps improve soil fertility.
- Food and beverage industry: Magnesium phosphate is sometimes used as a food additive in certain products. It may be added to enhance nutrient content or act as a pH regulator in certain food and beverage formulations.
- Pharmaceutical applications: Magnesium phosphate compounds are used in the pharmaceutical industry as excipients in drug formulations. They can serve as binders, fillers, or stabilizers in the production of tablets, capsules, and other dosage forms.
- Water treatment: Magnesium phosphate compounds are utilized in water treatment processes to control scale and corrosion in industrial systems. They can help prevent the formation of deposits and protect the integrity of pipes and equipment.
- Animal nutrition: Magnesium phosphate can be included in animal feed to provide essential minerals for livestock and poultry. It contributes to their overall health, growth, and development.
Physical Properties of Magnesium Phosphate Formula
- Appearance: White solid
- Crystalline Structure: Various crystalline forms exist, including hexagonal and monoclinic.
- Melting Point: The melting point can range from approximately 1,100 to 1,400 degrees Celsius.
- Density: The density of magnesium phosphate varies depending on the specific form but is generally around 2.8 to 3.2 grams per cubic centimeter.
- Solubility: Magnesium phosphate is typically insoluble in water, but its solubility can depend on the specific form and conditions.
- Hygroscopicity: It may exhibit some degree of hygroscopic behavior, meaning it can absorb moisture from the air.
- Stability: Magnesium phosphate is stable under normal conditions but may decompose at high temperatures.
Chemical Properties of Magnesium Phosphate Formula
- Acid-Base Reaction: Magnesium phosphate can react with acids to form corresponding salts and release phosphoric acid. For example, it reacts with hydrochloric acid to produce magnesium chloride and phosphoric acid.
Mg3(PO4)2 + 6HCl → 2MgCl2 + 2H3PO4
- Decomposition: At high temperatures, magnesium phosphate can decompose to yield magnesium oxide and phosphorus pentoxide. Mg3(PO4)2 → 3MgO + P2O5
- Reaction with Metals: Magnesium phosphate can react with certain metals, such as aluminum, to form corresponding phosphates and release magnesium metal. For example, it reacts with aluminum to form aluminum phosphate and magnesium metal. 2Al + 3Mg3(PO4)2 → 6Mg + 2AlPO4
- Solubility: The solubility of magnesium phosphate varies depending on the specific form and conditions. Some forms, such as magnesium ammonium phosphate (struvite), are more soluble than others.
- Stability: Magnesium phosphate is generally stable under normal conditions but can undergo various reactions when exposed to specific reactants or conditions.
Conclusion
In conclusion, the formula for magnesium phosphate (Mg3(PO4)2) represents a compound composed of magnesium (Mg) cations and phosphate (PO4) anions. Magnesium phosphate is an inorganic compound that has various applications in different fields. It is commonly used as a dietary supplement and in the production of fertilizers for plant nutrition. Additionally, magnesium phosphate can be utilized in the pharmaceutical industry for the formulation of medicines and as an additive in food and beverages. Its chemical formula reflects the specific combination of elements that make up this compound, providing the basis for its properties and uses.
Solved Examples on Magnesium Phosphate Formula
Example 1: Calculate the mass of magnesium phosphate (Mg3(PO4)2) formed when 25.0 g of magnesium nitrate (Mg(NO3)2) reacts with excess phosphoric acid (H3PO4).
Solution:
- Write the balanced chemical equation for the reaction:
- 3 Mg(NO3)2 + 2 H3PO4 → Mg3(PO4)2 + 6 HNO3
- Determine the molar mass of the reactant and product:
Molar mass of Mg(NO3)2 = 148.31 g/mol
Molar mass of H3PO4 = 97.99 g/mol
Molar mass of Mg3(PO4)2 = 262.86 g/mol
- Calculate the moles of magnesium nitrate: moles of Mg(NO3)2 = mass / molar mass moles of Mg(NO3)2 = 25.0 g / 148.31 g/mol = 0.1685 mol
- According to the balanced equation, the ratio between Mg(NO3)2 and Mg3(PO4)2 is 3:1. Therefore, the moles of magnesium phosphate formed are also 0.1685 mol.
- Calculate the mass of Mg3(PO4)2 = 0.1685 mol × 262.86 g/mol = 44.33 g
Therefore, the mass of magnesium phosphate formed is 44.33 g.
Example 2: A 0.25 M solution of magnesium phosphate (Mg3(PO4)2) is prepared by dissolving a certain amount of magnesium phosphate in water. Calculate the number of moles of magnesium phosphate dissolved in 500 mL of the solution.
Solution:
- Determine the volume of the solution in liters: volume = 500 mL = 500/1000 L = 0.5 L
- Calculate the number of moles using the formula: moles = concentration × volume moles = 0.25 mol/L × 0.5 L = 0.125 mol
Therefore, there are 0.125 moles of magnesium phosphate dissolved in 500 mL of the solution.
Frequently Asked Questions on Magnesium Phosphate Formula
1: What is another name for magnesium phosphate?
Answer: Another name for magnesium phosphate is Newberyite. Magnesium phosphate is also known as Trimagnesium phosphate.
2: Does magnesium phosphate react with water?
Answer: Magnesium phosphate can react with water, although the reaction is generally slow and limited. Magnesium phosphate is not highly soluble in water, and its solubility depends on the specific form and conditions. In general, magnesium phosphate compounds can dissociate in water to release magnesium ions (Mg2+) and phosphate ions (PO43-).
Mg3(PO4)2 + 6H2O → 2H3PO4 + 3Mg(OH)2
3: Is magnesium phosphate insoluble?
Answer: In general, magnesium phosphate is considered to be relatively insoluble in water. However, the solubility can be influenced by factors such as pH, temperature, and concentration of the solution. Under normal conditions, magnesium phosphate tends to have limited solubility, resulting in a cloudy or slightly turbid solution.
4: What is magnesium phosphate made of?
Answer: Magnesium phosphate is made up of magnesium ions (Mg2+) and phosphate ions (PO43-). The chemical formula for magnesium phosphate varies depending on the specific form or compound. One common form is magnesium phosphate tribasic, which has the chemical formula Mg3(PO4)2. In this compound, three magnesium ions are combined with two phosphate ions. Another example is magnesium ammonium phosphate, also known as struvite, which has the chemical formula MgNH4PO4·6H2O. This compound contains magnesium ions, ammonium ions (NH4+), phosphate ions, and water molecules. Magnesium phosphate can also exist in other forms and compounds, each with its own unique chemical composition.
5: What is the colour of magnesium phosphate precipitate?
Answer: The colour of magnesium phosphate precipitate can vary depending on the specific conditions and impurities present. In its pure form, magnesium phosphate is typically white or off-white. However, if impurities are present, it can exhibit different colors such as light yellow or pale pink. The color may also change with variations in pH or other environmental factors. It’s important to note that the color of the precipitate is not a definitive characteristic of magnesium phosphate and can be influenced by various factors.
6: What is magnesium phosphate used to treat?
Answer: Magnesium phosphate is commonly used as a dietary supplement to address magnesium deficiency in the body. Magnesium plays a crucial role in various physiological processes, including muscle and nerve function, energy production, and the maintenance of healthy bones. Supplementing with magnesium phosphate can help restore adequate magnesium levels in individuals who have a deficiency or are at risk of developing one. Additionally, magnesium phosphate is sometimes used as an ingredient in antacids to help relieve symptoms of acid indigestion or heartburn. However, it’s important to note that specific medical conditions and treatments should be discussed with a healthcare professional for personalized advice.
7: What is magnesium phosphate formula?
Answer: The formula for magnesium phosphate is Mg3(PO4)2.
8: Is Magnesium phosphate used in food?
Answer: Magnesium phosphate is not commonly used as a direct food additive. However, it can indirectly contribute to the nutritional content of certain foods that naturally contain magnesium and phosphate compounds. Magnesium and phosphate are essential nutrients for the human body, and they can be obtained from various food sources such as whole grains, nuts, seeds, legumes, and leafy green vegetables. These foods naturally contain magnesium and phosphate compounds, including magnesium phosphate, which play important roles in maintaining overall health and supporting various biological processes in the body.




