Nitrogen Dioxide Formula
Introduction
Nitrogen dioxide (NO2) is a chemical compound composed of nitrogen and oxygen. It is a reddish-brown gas with a pungent odor and is known for its role in air pollution. Nitrogen dioxide is formed as a result of the combustion of fossil fuels, industrial processes, and certain chemical reactions. It is a highly reactive compound and plays a significant role in atmospheric chemistry and the formation of smog. Nitrogen dioxide is considered harmful to human health and the environment, and efforts are made to control its emissions and reduce its impact on air quality.
Structural Formula of Nitrogen Dioxide
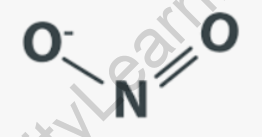
In this structure, the nitrogen atom (N) is connected to two oxygen atoms (O) by double bonds, and there is a lone pair of electrons on the nitrogen atom. The molecule has a bent or V-shape geometry.
Uses of Nitrogen Dioxide
- Industrial Processes: Nitrogen dioxide is used in various industrial processes, such as the production of nitric acid and other chemicals. It is an important precursor for the synthesis of nitrogen-based compounds used in fertilizers, explosives, and pharmaceuticals.
- Air Pollution Monitoring: Nitrogen dioxide is a major component of air pollution, primarily from combustion processes in vehicles, power plants, and industrial emissions. Monitoring levels of nitrogen dioxide in the atmosphere helps assess air quality and identify areas with high pollution levels.
- Laboratory Reagent: Nitrogen dioxide is used as a reagent in laboratory experiments and chemical synthesis. It can participate in redox reactions, acting as an oxidizing agent or a source of nitro groups in organic synthesis.
- Rocket Propellant: Nitrogen dioxide is utilized as an oxidizer in rocket propellants. When combined with a fuel, it undergoes a chemical reaction that produces thrust and propels the rocket.
- Metal Cleaning and Etching: Nitrogen dioxide is sometimes used for metal cleaning and etching processes in industries such as electronics and semiconductor manufacturing. It can effectively remove contaminants from metal surfaces and prepare them for further processing.
Physical properties of Nitrogen Dioxide Formula
- Molecular formula: NO2
- Molecular weight: 46.01 g/mol
- Appearance: Reddish-brown gas
- Odour: Pungent, choking odor
- Melting point: -11.2 °C (11.8 °F)
- Boiling point: 21.2 °C (70.2 °F)
- Density: 1.88 g/L at 25 °C (77 °F) and 1 atm pressure
- Solubility: Moderately soluble in water, forming nitric acid (HNO3) and nitrous acid (HNO2).
- Vapour pressure: 85.1 kPa (640 mmHg) at 25 °C (77 °F)
- Physical state: Nitrogen dioxide is a gas at standard temperature and pressure (STP).
Chemical Properties of Nitrogen Dioxide Formula
- Redox Reactions: Nitrogen dioxide is a reactive compound that participates in various redox reactions. It can act as both an oxidizing agent and a reducing agent. In certain reactions, it can accept electrons and get reduced to nitric oxide (NO). Conversely, it can donate electrons and get oxidized to higher oxides of nitrogen, such as nitrogen trioxide (N2O3) or nitrogen tetroxide (N2O4).
- Acidic Nature: Nitrogen dioxide can dissolve in water to form nitric acid (HNO3), making the solution acidic. It reacts with water to produce nitric acid and nitrous acid (HNO2). This property is due to the liberation of hydrogen ions (H+) from the nitric acid formed.
- Reaction with Alkalis: Nitrogen dioxide can react with alkalis, such as sodium hydroxide (NaOH), to form nitrites. The reaction produces sodium nitrite (NaNO2) and water.
- Formation of Nitrate Salts: Nitrogen dioxide can combine with oxygen to form higher oxides of nitrogen, such as nitrogen pentoxide (N2O5). These higher oxides readily dissolve in water to produce nitric acid, which can then react with various metals or bases to form nitrate salts.
- Atmospheric Reaction: In the atmosphere, nitrogen dioxide participates in various reactions, including those with hydrocarbons and other pollutants, leading to the formation of secondary pollutants such as ozone (O3) and particulate matter.
Conclusion
Nitrogen dioxide (NO2) is a versatile chemical compound with various applications in industry, laboratory settings, and rocket propulsion. It is commonly used in the production of nitric acid, as a reagent in chemical synthesis, for air pollution monitoring, and as an oxidizer in rocket propellants. However, it is crucial to handle nitrogen dioxide with caution due to its toxic nature. Exposure to high concentrations of nitrogen dioxide can be harmful to human health and the environment. Proper safety measures should always be taken when working with or around nitrogen dioxide to mitigate its potential risks.
Solved Examples on Nitrogen Dioxide Formula
Example 1: Stoichiometry and Mole Calculation
How many moles of nitrogen dioxide are present in 15 grams of NO2?
Solution: To determine the number of moles of nitrogen dioxide, we need to use its molar mass.
Molar mass of NO2 = (Molar mass of N) + (2 × Molar mass of O)
= (14.01 g/mol) + (2 × 16.00 g/mol)
= 14.01 g/mol + 32.00 g/mol
= 46.01 g/mol
Number of moles = Mass / Molar mass
= 15 g / 46.01 g/mol ≈ 0.326 moles
Therefore, there are approximately 0.326 moles of nitrogen dioxide present in 15 grams of NO2.
Example 2: Gas Stoichiometry
Nitrogen dioxide reacts with oxygen to form nitrogen tetroxide. If 2 moles of nitrogen dioxide react completely, how many moles of nitrogen tetroxide will be produced?
Solution: The balanced chemical equation for the reaction is:
2NO2 + O2 → 2N2O4
According to the balanced equation, 2 moles of nitrogen dioxide react to form 2 moles of nitrogen tetroxide.
Therefore, if 2 moles of nitrogen dioxide react completely, 2 moles of nitrogen tetroxide will be produced.
Frequently Asked Questions on Nitrogen Dioxide Formula
1: How is NO2 formula formed?
Answer: The NO2 formula is formed based on the valence electrons of the nitrogen (N) and oxygen (O) atoms involved.
Nitrogen, located in Group 15 of the periodic table, has 5 valence electrons, while oxygen, in Group 16, has 6 valence electrons. To achieve a stable electron configuration, nitrogen needs 3 more electrons, and oxygen needs 2 more electrons.
In the formation of nitrogen dioxide (NO2), one oxygen atom shares two electrons with nitrogen, forming a double bond. This contributes 4 electrons to the nitrogen atom, fulfilling its requirement for 3 more electrons.
The remaining oxygen atom forms a single bond with nitrogen, sharing 2 electrons. As a result, each oxygen atom contributes 1 electron to the nitrogen atom, fulfilling the nitrogen’s electron requirement.
Thus, the nitrogen atom in NO2 has achieved an octet (8 electrons) by forming a double bond with one oxygen atom and a single bond with another oxygen atom. The resulting formula for nitrogen dioxide is NO2.
2: Is NO2 stable or unstable?
Answer: Nitrogen dioxide (NO2) is relatively unstable and reactive under normal conditions. It is a highly reactive and toxic gas that readily undergoes various chemical reactions. The instability of NO2 arises from the presence of an unpaired electron on the nitrogen atom, making it susceptible to further reactions.
Due to its reactivity and potential health hazards, NO2 should be handled with caution. It is important to minimize exposure to NO2 and control its release into the environment to protect human health and maintain air quality.
3: Does NO2 cause acid rain?
Answer: Nitrogen dioxide (NO2) is not a direct contributor to the formation of acid rain. However, it does play a role in the formation of nitric acid (HNO3), which is one of the components of acid rain.
When NO2 is released into the atmosphere, it can react with oxygen (O2) to form nitrogen trioxide (NO) through a process called photolysis, which is facilitated by sunlight. Nitrogen trioxide can further react with oxygen to form nitrogen dioxide again. This is part of a cyclic process known as the nitrogen oxide cycle.
In the presence of water vapor and other atmospheric pollutants, nitrogen dioxide (NO2) can also react with water to form nitric acid (HNO3) through the following reaction:
3NO2 + H2O → 2HNO3 + NO
Nitric acid (HNO3) is highly soluble in water and can contribute to the acidity of rainwater. When nitric acid combines with other acidic compounds, such as sulfuric acid (H2SO4), they can collectively contribute to the formation of acid rain.
Therefore, while NO2 itself is not directly responsible for causing acid rain, it is involved in reactions that lead to the formation of nitric acid, which can contribute to the acidity of rainfall.
4: Does NO2 form naturally?
Answer: Nitrogen dioxide, or NO2, is a gaseous air pollutant composed of nitrogen and oxygen and is one of a group of related gases called nitrogen oxides, or NOx. NO2 forms when fossil fuels such as coal, oil, gas or diesel are burned at high temperatures. It is also formed through Lightning, Microbial Activities, Forest Fires, Volcanic Activity, and Biological Processes.
5: Does NO2 form ozone?
Answer: NO2 contributes to formation of several other air pollutants, including ozone (O3), nitric acid (HNO3), and nitrate (NO3-) -containing particles that also form through photochemical reactions.
6: Why NO2 is an air pollutant?
Answer: NO2 (nitrogen dioxide) is considered an air pollutant primarily due to its contribution to the formation of smog and its harmful effects on human health. Here are a few reasons why NO2 is considered an air pollutant:
- Smog Formation: NO2 reacts with volatile organic compounds (VOCs) in the presence of sunlight to form ground-level ozone, which is a major component of smog. Smog can have detrimental effects on air quality and human health.
- Respiratory Irritant: NO2 is an irritant to the respiratory system. Inhalation of high levels of NO2 can cause respiratory symptoms such as coughing, wheezing, and shortness of breath. It can also exacerbate existing respiratory conditions like asthma.
- Nitric Acid Formation: NO2 can react with atmospheric moisture to form nitric acid (HNO3), which contributes to acid rain. Acid rain can damage ecosystems, harm vegetation, and corrode buildings and infrastructure.
- Environmental Impacts: NO2 emissions contribute to the formation of fine particulate matter (PM2.5), which is harmful to both human health and the environment. PM2.5 can penetrate deep into the lungs and cause respiratory and cardiovascular problems.
- Ozone Layer Depletion: In the upper atmosphere, NO2 can react with ozone (O3), contributing to the depletion of the ozone layer, which protects the Earth from harmful ultraviolet (UV) radiation.
7: Is NO2 an inert gas?
Answer: No, NO2 (nitrogen dioxide) is not an inert gas. Inert gases, also known as noble gases, include elements such as helium, neon, argon, krypton, xenon, and radon. These gases are characterized by their low reactivity and tendency to exist as single atoms, which makes them chemically stable.
On the other hand, NO2 is a highly reactive gas. It is formed through the oxidation of nitrogen monoxide (NO) in the presence of oxygen in the atmosphere. NO2 readily participates in chemical reactions, particularly in the formation of smog and the production of nitric acid (HNO3).
8: What is nitrogen dioxide used for?
Answer: Nitrogen dioxide (NO2) has various uses in industrial and laboratory settings. One of its primary applications is in the production of nitric acid (HNO3), which is a key ingredient in the manufacturing of fertilizers, explosives, and various chemicals. NO2 is also used as an oxidizing agent in certain chemical reactions and as a catalyst in some industrial processes. Additionally, NO2 is utilized in the calibration of gas detection instruments and air quality monitoring systems. However, it’s important to note that NO2 is primarily considered an air pollutant and its industrial uses are regulated to minimize environmental and health risks.




