Table of Contents
Introduction Potassium Carbonate Formula
Potassium Carbonate, with the chemical formula K2CO3, is an inorganic compound composed of potassium ions carbonate ions. It is a white, crystalline solid that is highly soluble in water. Potassium Carbonate is commonly used in various industries and applications, including glass manufacturing, soap production, food processing, and as a buffering agent in chemical laboratories. It is also known by other names such as potash carbonate or pearl ash. Its formula represents the ratio of potassium to carbonate ions in the compound, indicating that it contains two potassium ions for every carbonate ion.
Structural Formula of Potassium Carbonate Formula
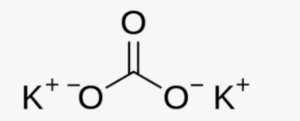
The structural formula of Potassium Carbonate (K2CO3) consists of two potassium (K) atoms bonded to a carbonate ion (CO3). The carbonate ion consists of one carbon atom (C) bonded to three oxygen atoms (O).
Uses of Potassium Carbonate
- Glass production: Potassium carbonate is a key ingredient in the manufacturing of glass. It helps improve the melting properties and stability of glass, making it clearer and less prone to cracking.
- Soap and detergent production: Potassium carbonate is used in the production of liquid soaps and detergents. It acts as a pH regulator and helps enhance the cleaning properties of these products.
- Food industry: Potassium carbonate is sometimes used as a food additive in certain applications. It can act as a leavening agent in baking, helping dough rise and creating a lighter texture in baked goods. It is also used as a pH regulator in food and beverage formulations.
- pH adjustment: Potassium carbonate is used to adjust the pH of various products and solutions. It can act as a buffering agent, maintaining a stable pH level in certain chemical processes or industrial applications.
- Agriculture: Potassium carbonate is used as a source of potassium in agricultural practices. It can be added to soils to replenish potassium levels and improve crop yields.
- Fire extinguishers: Potassium carbonate is a component of certain types of fire extinguishers. It functions by inhibiting chemical reactions and suppressing flames in specific fire classes.
- Photography: In black and white photography, potassium carbonate is used as a chemical agent in the development process.
Physical properties of Potassium Carbonate Formula
- Molar mass: Potassium Carbonate Molar Mass is 138.21 g/mol, computed from the potassium carbonate formula.
- Density: The substance has a density of 2.43 g/cm3.
- Hygroscopicity: Its status as a white hygroscopic solid—i.e., its ability to hold onto water molecules—has been well described.
- Melting point and Boiling point: 891°C is the melting point. It has no boiling point because it breaks down when heated to extremely high levels.
- Solubility: In addition to being soluble in water, it is also soluble in a methanol solvent and indissoluble in an ether and acetone solvent.
Chemical Properties of Potassium Carbonate Formula
- Solubility: Potassium carbonate is highly soluble in water. It readily dissolves and forms a clear, colorless solution.
- Basicity: Potassium carbonate is a strong base. It undergoes hydrolysis in water, releasing hydroxide ions (OH-) and raising the pH of the solution.
- Reactivity: Potassium carbonate is reactive with acids, forming potassium salts and carbon dioxide gas. The reaction can be represented as follows: K2CO3 + 2HCl -> 2KCl + CO2 + H2O
- Deliquescence: Potassium carbonate is hygroscopic, meaning it can absorb moisture from the air and become damp or dissolve. It has a tendency to form a liquid solution when exposed to humid conditions.
- Thermal decomposition: At high temperatures, potassium carbonate decomposes to form potassium oxide (K2O) and carbon dioxide gas. This process is known as thermal decomposition. 2K2CO3 -> 2K2O + 3CO2
- Use as a reagent: Potassium carbonate is commonly used in various chemical reactions, such as in the production of soap, glass, and ceramics. It is also used as a buffering agent and pH regulator in certain applications.
Conclusion
In conclusion, the formula for potassium carbonate (K2CO3) represents a compound composed of potassium (K) cations and carbonate (CO3) anions. Potassium carbonate is an important chemical compound with a wide range of applications. It is commonly used as a source of potassium in fertilizers, contributing to plant growth and development. In addition, potassium carbonate finds use in various industries such as glass manufacturing, soap production, and as a pH regulator in water treatment processes. Its chemical formula reflects the specific combination of elements that make up this compound, which determines its properties and applications.
Solved Examples on Potassium Carbonate Formula
Example 1: Calculate the molar mass of potassium carbonate (K2CO3).
Solution: The molar mass of potassium (K) is approximately 39.10 g/mol, and the molar mass of carbon (C) is approximately 12.01 g/mol. The molar mass of oxygen (O) is approximately 16.00 g/mol.
Molar mass of K2CO3 = (2 * molar mass of K) + molar mass of C + (3 * molar mass of O)
= (2 * 39.10 g/mol) + 12.01 g/mol + (3 * 16.00 g/mol)
= 78.20 g/mol + 12.01 g/mol + 48.00 g/mol = 138.21 g/mol
Therefore, the molar mass of potassium carbonate (K2CO3) is approximately 138.21 g/mol.
Example 2: What volume of carbon dioxide gas (CO2) at STP (standard temperature and pressure) will be produced by the complete reaction of 100 g of potassium carbonate (K2CO3)?
Solution: We need to use the balanced chemical equation for the reaction of potassium carbonate with an acid:
K2CO3 + 2HCl -> 2KCl + CO2 + H2O
From the balanced equation, we can see that 1 mole of K2CO3 produces 1 mole of CO2.
First, we need to determine the number of moles of K2CO3 in 100 g:
Number of moles of K2CO3 = Mass of K2CO3/ Molar mass of K2CO3
= 100 g / 138.21 g/mol ≈ 0.723 mol
Since the molar ratio between K2CO3 and CO2 is 1:1, the number of moles of CO2 produced will also be 0.723 mol.
At STP, 1 mole of any ideal gas occupies 22.4 L of volume. Therefore, the volume of CO2 gas produced is:
Volume of CO2 gas = Number of moles of CO2 * 22.4 L/mol
= 0.723 mol * 22.4 L/mol ≈ 16.19 L
Therefore, approximately 16.19 liters of carbon dioxide gas will be produced at STP by the complete reaction of 100 g of potassium carbonate (K2CO3).
Frequently Asked Questions on Potassium Carbonate Formula
Is potassium carbonate a salt or acid?
Potassium carbonate is a salt.
What is K2CO3 used for?
K2CO3, also known as potassium carbonate, is used in various applications, including as a fertilizer, in soap making, and to regulate pH in chemical processes.
What is the formula for potassium carbonate?
The formula for potassium carbonate is K2CO3.
What is the use of K2CO3?
K2CO3 is used for multiple purposes, such as in agriculture as a fertilizer, in soap production, and as a pH regulator in industrial processes.
Is potassium carbonate safe for humans?
Potassium carbonate is generally safe for humans when used in accordance with recommended guidelines. However, it should be handled with care and not ingested in its pure form.
How do you make potassium carbonate formula?
The formula for potassium carbonate (K2CO3) can be determined based on the chemical composition of two potassium ions (K+) and one carbonate ion (CO3^2-).
How do you write K2CO3?
To write K2CO3, simply use the chemical symbols for the elements: K for potassium and CO3 for carbonate, with the subscripts indicating the number of each element in the compound.








