Potassium chlorate is an inorganic compound with the chemical formula KClO3. It is composed of a potassium cation (K+) and a chlorate anion (ClO3-).
Potassium chlorate has several applications. It is commonly used in pyrotechnics to generate oxygen for combustion reactions. It is also used in chemical laboratories as an oxidizing agent and in the manufacture of matches, dyes, and disinfectants.
Structural Formula of Potassium Chlorate Formula
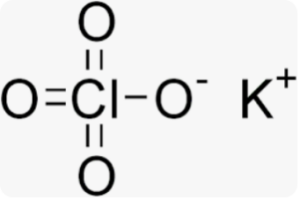
The formula KClO3 indicates that each potassium chlorate molecule consists of one potassium ion (K+) and one chlorate ion (ClO3-).
Uses of Potassium Chlorate
- Oxygen source: Potassium chlorate is widely used as an oxygen source in various applications. It can be used in the production of oxygen gas for laboratory experiments, as well as in chemical reactions that require an oxygen supply.
- Matches and fireworks: Potassium chlorate is a key ingredient in the composition of matches and fireworks. It serves as an oxidizer, providing the necessary oxygen to support combustion and create the desired effects in pyrotechnic displays.
- Explosives: Potassium chlorate is used in the production of certain types of explosives, such as flash powders and blasting agents. Its ability to release oxygen rapidly during decomposition makes it valuable in explosive formulations.
- Laboratory reagent: Potassium chlorate is used as a laboratory reagent in various chemical experiments and reactions. It can act as an oxidizing agent or a source of oxygen in specific reactions.
- Weed control: Potassium chlorate has been used as an herbicide to control weeds in certain agricultural and horticultural practices. However, its use for this purpose has become less common due to safety concerns and the availability of alternative herbicides.
Physical properties of Potassium Chlorate Formula
- Appearance: Potassium chlorate is a white crystalline solid. It typically forms small, colorless or white crystals that resemble fine powder or granules.
- Density: The density of potassium chlorate varies with temperature and pressure. At room temperature and standard atmospheric pressure, its density is approximately 2.32 grams per cubic centimeter (g/cm³).
- Melting Point: Potassium chlorate has a relatively high melting point. It melts at around 356 degrees Celsius (673 degrees Fahrenheit). The melting process is endothermic, requiring heat input to transition from a solid to a liquid state.
- Solubility: Potassium chlorate is highly soluble in water. It dissolves readily in water, forming a clear solution. The solubility increases with temperature, meaning that more potassium chlorate can dissolve in hot water compared to cold water.
- Crystal Structure: Potassium chlorate crystallizes in a monoclinic crystal system. Its crystals have a layered structure, with potassium cations (K+) and chlorate anions (ClO3-) arranged in a repeating pattern.
- Stability: Potassium chlorate is stable under normal conditions, but it can decompose when exposed to heat or certain catalysts. This decomposition reaction releases oxygen gas and leaves behind potassium chloride (KCl) as a residue.
- Odor and Taste: Potassium chlorate is odorless and tasteless.
- Hygroscopicity: Potassium chlorate has hygroscopic properties, meaning it can absorb moisture from the surrounding environment. It can become damp or dissolve in humid conditions.
- Conductivity: Potassium chlorate is a poor conductor of electricity in its solid state. However, when dissolved in water or in the molten state, it can conduct electricity due to the dissociation of ions.
Chemical Properties of Potassium Chlorate Formula
- Oxidizing Agent: Potassium chlorate is a powerful oxidizing agent. It can transfer oxygen to support combustion reactions. When heated, it decomposes to release oxygen gas, which can enhance the combustion of other substances.
- Thermal Decomposition: Potassium chlorate undergoes thermal decomposition when heated strongly. This decomposition reaction produces potassium chloride (KCl) and oxygen gas (O2). The reaction is highly exothermic and can be initiated by a flame or a catalyst.
2KClO3 -> 2KCl + 3O2
- Reaction with Reducing Agents: Potassium chlorate can react vigorously with reducing agents, such as organic compounds or certain metals, due to its oxidizing nature. It can provide oxygen to these substances, resulting in combustion or other chemical reactions.
- Reaction with Acids: Potassium chlorate reacts with strong acids to produce chlorine gas (Cl2) along with other products. The reaction is highly exothermic and can be dangerous. An example of this reaction is:
2KClO3 + 2HCl -> 2KCl + Cl2 + H2O
- Decomposition by Light: Potassium chlorate can decompose slowly when exposed to light, although the reaction is not as rapid as thermal decomposition. The decomposition by light primarily occurs in the presence of impurities or certain sensitizers.
- Use in Oxygen Supply: Potassium chlorate has been used in various applications as a source of oxygen, such as in the manufacture of oxygen masks and oxygen candles.
- Use in Pyrotechnics: Potassium chlorate is commonly used in pyrotechnics and fireworks due to its ability to release oxygen and support combustion reactions.
- Disinfectant Properties: Potassium chlorate exhibits some disinfectant properties. It has been used in certain disinfection applications, although its use has decreased due to safety concerns.
Conclusion
In conclusion, the formula for potassium chlorate (KClO3) represents a compound composed of potassium (K) cations and chlorate (ClO3) anions. Potassium chlorate has several important uses in various industries and applications. It is commonly used as an oxidizing agent in chemical processes, fireworks, and explosives manufacturing. Additionally, potassium chlorate is utilized in the production of matches, dyes, and disinfectants. Its chemical formula reflects the specific combination of elements that make up this compound, which determines its properties and reactivity.
Solved Examples on Potassium Chlorate Formula
Example 1: Calculate the number of moles of oxygen gas (O2) released by the complete decomposition of 100 grams of potassium chlorate (KClO3).
Solution: The balanced chemical equation for the decomposition of potassium chlorate is:
2KClO3 -> 2KCl + 3 O2
From the equation, we can see that 2 moles of potassium chlorate decompose to produce 3 moles of oxygen gas.
First, we need to determine the number of moles of potassium chlorate in 100 grams:
Number of moles of KClO3 = Mass of KClO3 / Molar mass of KClO3
The molar mass of KClO3 can be calculated as follows:
Molar mass of K = 39.10 g/mol
Molar mass of Cl = 35.45 g/mol
Molar mass of O = 16.00 g/mol
Molar mass of KClO3 = (39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol)
= 39.10 g/mol + 35.45 g/mol + 48.00 g/mol
= 122.55 g/mol
Number of moles of KClO3 = 100 g / 122.55 g/mol ≈ 0.816 mol
Since the molar ratio between KClO3 and O2 is 2:3, the number of moles of O2 produced will be:
Number of moles of O2 = (3/2) * Number of moles of KClO3 = (3/2) * 0.816 mol ≈ 1.224 mol
Therefore, approximately 1.224 moles of oxygen gas will be released by the complete decomposition of 100 grams of potassium chlorate.
Example 2: What mass of potassium chloride (KCl) will be produced from the decomposition of 25 grams of potassium chlorate (KClO3)?
Solution: Using the balanced chemical equation for the decomposition of potassium chlorate:
2 KClO3 -> 2KCl + 3O2
From the equation, we can see that 2 moles of potassium chlorate decompose to produce 2 moles of potassium chloride.
First, we need to determine the number of moles of potassium chlorate in 25 grams:
Number of moles of KClO3 = Mass of KClO3 / Molar mass of KClO3
The molar mass of KClO3 is 122.55 g/mol (as calculated in Example 1).
Number of moles of KClO3 = 25 g / 122.55 g/mol ≈ 0.204 mol
Since the molar ratio between KClO3 and KCl is 2:2, the number of moles of KCl produced will be the same as the number of moles of KClO3:
Number of moles of KCl = Number of moles of KClO3 ≈ 0.204 mol
To determine the mass of potassium chloride produced, we need to multiply the number of moles of KCl by its molar mass:
Mass of KCl = Number of moles of KCl * Molar mass of KCl
The molar mass of KCl is approximately 74.55 g/mol.
Mass of KCl = 0.204 mol * 74.55 g/mol ≈ 15.19 g
Frequently Asked Questions on Potassium Chlorate Formula
1: What is another name for potassium chlorate?
Answer: Another name for potassium chlorate is “potassic chlorate.” The term “potassic” is derived from the Latin word “potassa,” which means potash or potassium carbonate. The use of “potassic” in the name emphasizes the presence of potassium in the compound. Therefore, both “potassium chlorate” and “potassic chlorate” refer to the same chemical compound with the formula KClO3.
2: Is potassium chlorate a strong oxidizing agent?
Answer: Potassium chlorate (KClO3) is a strong oxidizing agent that has a wide variety of uses. It is or has been a component of explosives, fireworks, safety matches, and disinfectants.
3: Is potassium chlorate banned in India?
Answer: Potassium chlorate has been a chemical banned by the Petroleum and Explosives Safety Organisation (PESO) in fireworks (in 1992) because of the extreme hazards involved and the high noise levels it can generate.
4: What is the source of potassium chlorate?
Answer: On the industrial scale, potassium chlorate is produced by the salt metathesis reaction of sodium chlorate and potassium chloride:
NaClO3 + KCl → NaCl + KClO.
We can also make Potassium chlorate from
- Chlorine bleach
- Potassium chloride (sold as a salt substitute)
5: Why potassium chlorate is used in gargles?
Answer: Potassium chlorate are used as a constituent of mouth washes, gargles, dentifrices and preparations for oral conditions such as stomatitis and mercurial poisoning.
6: Is KClO3 a gas?
Answer: No, potassium chlorate (KClO3) is not a gas at room temperature and normal atmospheric pressure. It is a solid compound that exists in crystalline form. Potassium chlorate has a white crystalline appearance and is typically found in the form of crystals or powder. It is highly soluble in water, meaning it readily dissolves in water to form a solution.
7: How potassium chlorate decompose?
Answer: Potassium chlorate (KClO3) decomposes when heated to high temperatures, releasing oxygen gas (O2) and leaving behind potassium chloride (KCl) as the residue. The decomposition reaction can be represented by the following equation:
2 KClO3 → 2 KCl + 3O2
When potassium chlorate is heated, it undergoes a chemical reaction that breaks down its molecular structure, resulting in the formation of potassium chloride and oxygen gas as products. The release of oxygen gas during this decomposition reaction makes potassium chlorate a useful compound in various applications, such as in fireworks and explosives. However, due to its potentially hazardous nature and the risk of spontaneous combustion, potassium chlorate has restricted or banned uses in many countries.
8: What happens when Potassium Chlorate is heated?
Answer: When potassium chlorate (KClO3) is heated, it undergoes a decomposition reaction. The heat provides the energy needed to break the bonds within the compound, resulting in the formation of new substances. The decomposition of potassium chlorate can be summarized by the following equation:
2 KClO3 (s) → 2KCl(s) + 3O2 (g)
In this reaction, solid potassium chlorate decomposes into solid potassium chloride (KCl) and oxygen gas (O2). The release of oxygen gas is a characteristic feature of this decomposition reaction.


