Potassium chromate is a chemical compound with the formula K2CrO4.
Potassium chromate (K2CrO4) is a chemical compound that belongs to the chromate family. It is an inorganic salt that is commonly used in various industrial and laboratory applications. The compound appears as bright yellow crystals or a yellow powder.
Potassium chromate is primarily known for its role as an important chemical reagent. It is widely used in chemical laboratories for analytical purposes, such as in titration reactions and as an indicator in various chemical tests. The compound is also utilized in the production of other chemicals, including dyes, pigments, and chrome plating.
The formula of Potassium Chromate
The chemical formula of potassium chromate is K2CrO4. It indicates that the compound contains two potassium ions (K+) and one chromate ion (CrO42-).
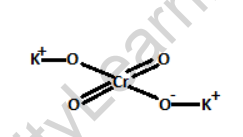
Structure of Potassium Chromate
Potassium chromate has a crystal lattice structure, with potassium cations (K+) and chromate anions (CrO42-) arranged in a three-dimensional pattern. The potassium ion is positively charged, while the chromate ion is negatively charged.
Physical properties of Potassium Chromate
– Appearance: Potassium chromate typically appears as bright yellow crystals or a yellow powder.
– Molecular weight: The molar mass of potassium chromate is 194.19 g/mol.
– Solubility: Potassium chromate is soluble in water, meaning it readily dissolves in water to form a yellow solution.
Chemical properties of Potassium Chromate
– Oxidizing Agent: Potassium chromate is a strong oxidizing agent, meaning it can cause the oxidation of other substances by accepting electrons.
– Reactivity: It can react with reducing agents and combustible materials, making it potentially hazardous when not handled properly.
Uses of Potassium Chromate
– Laboratory Reagent: Potassium chromate is commonly used as a reagent in laboratory settings for various chemical analyses, including titrations and redox reactions.
– Dye Production: It is used in the production of certain dyes, pigments, and inks due to its yellow color.
– Wood Preservation: Potassium chromate has been used as a wood preservative to protect against decay and insect infestation. However, its use in this application has been restricted or banned in many countries due to environmental and health concerns.
Potassium Chromate (K2CrO4) Conclusion
It’s worth noting that potassium chromate should be handled with caution, as it is toxic and can pose environmental hazards. Proper safety measures and guidelines should be followed when working with this compound.
In addition to its industrial applications, potassium chromate has certain uses in the field of biology and medicine. It is employed in certain laboratory tests and medical procedures, such as determining blood glucose levels and identifying certain types of bacteria.
It is important to note that potassium chromate is a toxic substance and should be handled with caution. It poses health hazards if ingested, inhaled, or in contact with the skin or eyes. Proper safety precautions should be followed when working with this compound to ensure personal safety and prevent environmental contamination.
Solved examples on Potassium Chromate (K2CrO4)
Example 1: Calculate the number of moles of potassium chromate in 25 grams of K2CrO4.
Solution:
– Molar mass of K2CrO4 = (39.1 g/mol × 2) + (52.0 g/mol + 16.0 g/mol × 4)
= 194.19 g/mol
– Given mass of K2CrO4 = 25 grams
– Number of moles of K2CrO4 = Given mass / Molar mass
= 25 g / 194.19 g/mol
≈ 0.129 moles
Therefore, there are approximately 0.129 moles of potassium chromate in 25 grams of K2CrO4.
Example 2: How many grams of potassium chromate are required to prepare 500 mL of a 0.2 M K2CrO4 solution?
Solution:
– Molar mass of K2CrO4 = 194.19 g/mol
– Molarity (M) of K2CrO4 solution = 0.2 mol/L
– Volume of K2CrO4 solution = 500 mL = 0.5 L
– Number of moles of K2CrO4 required = Molarity × Volume
= 0.2 mol/L × 0.5 L
= 0.1 moles
– Mass of K2CrO4 required = Number of moles × Molar mass
= 0.1 moles × 194.19 g/mol
= 19.42 grams
Therefore, approximately 19.42 grams of potassium chromate are required to prepare 500 mL of a 0.2 M K2CrO4 solution.
These examples demonstrate the application of molar mass calculations, mole ratios, and concentration calculations in solving problems related to potassium chromate.
Frequently asked questions on Potassium Chromate
1: Why potassium chromate is used as an indicator?
Answer: Potassium chromate (K2CrO4) is used as an indicator in various chemical reactions and analytical processes due to its distinct color change at specific pH levels. The color change serves as a visual signal to indicate the endpoint or completion of a reaction. Here’s why potassium chromate is commonly used as an indicator:
– Acid-Base Titrations: Potassium chromate is frequently employed as an indicator in acid-base titrations. It undergoes a color change from yellow to orange when the pH of the solution shifts from acidic to slightly alkaline. The color transition occurs near a pH range of 5.0 to 6.8. This color change signals the equivalence point of the titration, indicating that the reaction has reached completion.
– Redox Reactions: Potassium chromate is also utilized as an indicator in certain redox (oxidation-reduction) reactions. It acts as a self-indicator, undergoing a color change during the course of the reaction. For instance, in the presence of a reducing agent, such as ferrous ions (Fe2+), the yellow potassium chromate solution turns to green due to the formation of chromium(III) ions (Cr3+).
2: Is potassium chromate diamagnetic or paramagnetic?
Answer: Potassium chromate (K2CrO4) is paramagnetic. Paramagnetic substances are those that contain unpaired electrons in their atomic or molecular orbitals, which results in the presence of magnetic properties. In the case of potassium chromate, the chromium (Cr) atom in its chemical structure has unpaired electrons in its d-orbitals. As a result, it exhibits paramagnetic behavior and can be attracted by an external magnetic field.
3: Why potassium chromate is used in Mohr method?
Answer: Potassium chromate (K2CrO4) is used in the Mohr method, which is a widely used titration technique to determine the concentration of chloride ions (Cl-) in a solution. Here’s why potassium chromate is employed in the Mohr method:
– Indicator: In the Mohr method, potassium chromate acts as an indicator to visually detect the endpoint of the titration. Initially, a known volume of the chloride-containing solution is titrated with a silver nitrate (AgNO3) solution of known concentration. As silver ions (Ag+) are added to the solution, they react with chloride ions to form a white precipitate of silver chloride (AgCl).
– Color Change: The role of potassium chromate is to provide a color change during the titration process. Initially, potassium chromate imparts a yellow color to the solution. As silver ions are added and chloride ions are consumed, the silver ions begin to react with the excess potassium chromate, forming a red-brown precipitate of silver chromate (Ag2CrO4). The appearance of this red-brown color signals the endpoint of the titration, indicating that all the chloride ions have reacted with the silver ions.
By using potassium chromate as an indicator, the endpoint of the titration can be easily identified, allowing for accurate determination of the chloride concentration. The Mohr method offers a relatively simple and cost-effective way to measure chloride ions in various samples, including water, biological fluids, and industrial processes.
4: What type of bond is potassium chromate?
Answer: Potassium chromate (K2CrO4) contains both ionic and covalent bonds. The compound consists of potassium ions (K+) and chromate ions (CrO42-). The potassium ion forms an ionic bond with the chromate ion, resulting in the overall ionic character of the compound.
The chromate ion (CrO42-) itself consists of a central chromium atom (Cr) bonded to four oxygen atoms (O) through covalent bonds. The oxygen atoms share electrons with the chromium atom, forming covalent bonds within the chromate ion.
Therefore, the bonding in potassium chromate involves ionic bonding between the potassium ion and the chromate ion, as well as covalent bonding within the chromate ion itself.
5: What is the use of potassium dichromate?
Answer: It is used in many applications as an oxidizing agent and is also used in the preparation of different products such as waxes, paints, glues, etc. Potassium dichromate is carcinogenic and highly toxic as a compound of hexavalent chromium.
6: What is the formula of potassium chromate?
Answer: The formula of potassium chromate is K2CrO4.
7: What is the difference between K2CrO4 and K2Cr2O7?
Answer: The main difference between K2CrO4 and K2Cr2O7 is the number of oxygen atoms present in the compound. K2CrO4 contains one chromium atom (Cr), two potassium atoms (K), and four oxygen atoms (O), whereas K2Cr2O7 contains one chromium atom (Cr), two potassium atoms (K), and seven oxygen atoms (O). This means that K2Cr2O7 has more oxygen atoms compared to K2CrO4.
8: What is the name of K2Cr2O7?
Answer: The name of K2Cr2O7 is potassium dichromate.


