Potassium Hydroxide Formula
Potassium hydroxide, also known as caustic potash, is a chemical compound with the formula KOH. It is an inorganic base and alkali metal hydroxide. Potassium hydroxide is highly soluble in water and forms a strong alkaline solution. It is a white, odorless solid that absorbs moisture from the air.
Structural Formula of Potassium Hydroxide
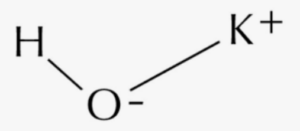
The formula for potassium hydroxide (KOH) consists of the potassium ion (K+) and the hydroxide ion (OH–). The hydroxide ion consists of one oxygen atom and one hydrogen atom with a charge of 1-. The potassium ion carries a charge of +1. When combined, the potassium ion and hydroxide ion form an ionic bond, resulting in the compound potassium hydroxide (KOH).
Uses of Potassium Hydroxide
- Industrial Cleaning: Potassium hydroxide is a powerful alkali and is used in various cleaning and degreasing applications. It can remove stubborn stains, grease, and oils from surfaces.
- Soap and Detergent Production: Potassium hydroxide is a key ingredient in the production of liquid soaps and detergents. It helps in saponification, the process of converting fats and oils into soap.
- pH Regulation: Potassium hydroxide is commonly used in laboratories and industries to adjust the pH of solutions. It is an alkaline substance and can be used to neutralize acidic solutions.
- Electrolyte in Batteries: Potassium hydroxide is used as an electrolyte in certain types of batteries, such as alkaline batteries. It helps facilitate the movement of ions between the battery’s electrodes.
- Food Processing: Potassium hydroxide is used in certain food processing applications, such as peeling fruits and vegetables. It helps in removing the outer skin or peel of produce.
- pH Control in Agriculture: Potassium hydroxide can be used in agriculture to adjust the pH of soil and water. It can be applied to fields or irrigation systems to modify the acidity or alkalinity of the growing environment.
- Chemical Manufacturing: Potassium hydroxide is used in the production of various chemicals, including potassium salts, dyes, fertilizers, and pharmaceuticals.
Physical properties of Potassium Hydroxide Formula
- Appearance: Potassium hydroxide is a white, odorless solid in its pure form. It is commonly available in the form of pellets, flakes, or granules.
- Solubility: Potassium hydroxide is highly soluble in water, and it readily dissolves to form a strong alkaline solution.
- Melting Point: The melting point of potassium hydroxide is approximately 360°C (680°F). At higher temperatures, it can undergo decomposition.
- Density: The density of solid potassium hydroxide is around 2.04 g/cm³.
- Hygroscopicity: Potassium hydroxide is hygroscopic, meaning it absorbs moisture from the air, and it can deliquesce (dissolve in its own water of hydration) if not properly stored.
- Corrosiveness: Potassium hydroxide is corrosive to many materials, including metals, and it can cause burns or irritations upon contact with the skin, eyes, or mucous membranes.
- Odour: Potassium hydroxide does not have a distinct odor, although it may have a slight alkaline smell.
Chemical Properties of Potassium Hydroxide Formula
- Strong Base: Potassium hydroxide is a strong base and readily dissociates in water to release hydroxide ions (OH–) which can react with acids to form water and salts.
- Neutralization Reactions: Potassium hydroxide can react with acids in neutralization reactions, forming water and potassium salts. These reactions are often exothermic.
Example: KOH + HCl → KCl + H2O
- Alkaline Properties: Potassium hydroxide solutions are highly alkaline due to the presence of hydroxide ions. They can react with acidic substances and neutralize them.
- Corrosive: Potassium hydroxide is highly corrosive and can react with various materials, including metals, causing them to corrode or dissolve.
- Saponification: Potassium hydroxide is commonly used in the process of saponification, where it reacts with fats or oils to produce potassium salts of fatty acids (soaps) and glycerol.
Example: KOH + Fat/Oil → Soap + Glycerol
- Dehydration Reactions: Potassium hydroxide can undergo dehydration reactions with certain compounds, removing water molecules and forming new products.
Example: 2KOH → K2O + H2O
- Reactivity with Carbon Dioxide: Potassium hydroxide reacts with carbon dioxide (CO2) to form potassium carbonate (K2CO3), a process called carbonation.
Example: 2KOH + CO2 → K2CO3 + H2O
Solved Examples on Potassium Hydroxide Formula
Example 1: Calculate the amount of Potassium Hydroxide required to neutralize 25 mL of 0.5 M Hydrochloric Acid (HCl) solution.
Solution:
The balanced chemical equation for the neutralization reaction between Potassium Hydroxide and Hydrochloric Acid is:
KOH + HCl → KCl + HO
From the equation, we can see that the stoichiometric ratio between KOH and HCl is 1:1. This means that 1 mole of KOH reacts with 1 mole of HCl.
Given that the volume of HCl solution is 25 mL (0.025 L) and its concentration is 0.5 M, we can calculate the number of moles of HCl:
Moles of HCl = Concentration × Volume = 0.5 M × 0.025 L = 0.0125 moles
Since the stoichiometric ratio is 1:1, the number of moles of KOH required will be the same.
Moles of KOH required = 0.0125 moles
To convert moles to grams, we need to know the molar mass of KOH:
Molar mass of KOH = 39.1 g/mol (K) + 16.0 g/mol (O) + 1.0 g/mol (H)
= 56.1 g/mol
Mass of KOH required = Moles of KOH required × Molar mass of KOH
= 0.0125 moles × 56.1 g/mol = 0.70125 grams
Therefore, approximately 0.701 grams of Potassium Hydroxide (KOH) is required to neutralize 25 mL of 0.5 M Hydrochloric Acid (HCl) solution.
Example 2: A 250 mL solution contains 35 grams of Potassium Hydroxide (KOH). Calculate the molarity of the solution.
Solution:
Molarity (M) is defined as moles of solute divided by the volume of the solution in liters.
Given that the mass of Potassium Hydroxide (KOH) is 35 grams and the volume of the solution is 250 mL (0.250 L), we need to calculate the moles of KOH:
Moles of KOH = Mass of KOH / Molar mass of KOH = 35 g / 56.1 g/mol
= 0.623 moles
Now we can calculate the molarity:
Molarity (M) = Moles of KOH / Volume of solution (in liters)
= 0.623 moles / 0.250 L
= 2.492 M
Therefore, the molarity of the Potassium Hydroxide (KOH) solution is approximately 2.492 M.
Frequently Asked Questions on Potassium Hydroxide Formula
1: What is the common name for potassium hydroxide formula?
Answer: The common name of Potassium Hydroxide is Caustic Potash.
2: What is the nature of KOH?
Answer: Potassium hydroxide (KOH) is a strong base. It dissociates completely in water to release hydroxide ions (OH–) which can accept protons (H+) and increase the concentration of hydroxide ions in solution. Therefore, KOH is considered a basic compound.
3: What are the hazards of KOH?
Answer: Potassium hydroxide (KOH) is a corrosive substance and can cause severe skin and eye damage upon contact. It is highly caustic and can cause burns. Inhalation of potassium hydroxide dust or mist can irritate the respiratory system and cause respiratory distress. Ingestion of KOH can lead to severe internal damage and is toxic. It is important to handle potassium hydroxide with care, wear appropriate protective equipment, and follow safety protocols to avoid the hazards associated with its use.
4: Which is stronger base KOH or NaOH?
Answer: Sodium hydroxide (NaOH) is generally considered a stronger base compared to potassium hydroxide (KOH). NaOH dissociates completely in water, releasing more hydroxide ions (OH-) per mole compared to KOH. This higher concentration of hydroxide ions makes NaOH a stronger base in terms of its alkalinity and reactivity.
5: What type of catalyst is KOH?
Answer: Potassium hydroxide (KOH) can act as a catalyst in certain reactions. It is commonly used as a catalyst in organic chemistry, particularly in processes such as esterification, transesterification, and dehydration reactions. In these reactions, KOH helps facilitate the reaction by increasing the rate of reaction without being consumed in the process. It typically acts as a base catalyst, abstracting a proton from a reactant and forming an intermediate species that leads to the desired product. However, it’s important to note that the specific type of catalyst can depend on the reaction conditions and the reactants involved.
6: What is potassium hydroxide used for?
Answer: Potassium hydroxide is commonly used in various industries and applications.
- It is used in the production of soaps, detergents, and other cleaning agents.
- It is also used in the manufacture of potassium salts, fertilizers, and dyes.
- In laboratories, potassium hydroxide is often used as a reagent for chemical reactions and as a pH adjuster.
7: Is KOH a base or acid?
Answer: Potassium hydroxide (KOH) is a strong base. It dissociates in water to release hydroxide ions (OH–) and potassium ions (K+). The hydroxide ions make the solution alkaline, hence classifying KOH as a base.
8: What is the pH of KOH?
Answer: The pH of a solution of potassium hydroxide (KOH) depends on its concentration. When a strong base like KOH completely dissociates in water, the resulting solution is highly alkaline with a pH greater than 7. However, the exact pH value will depend on the concentration of the KOH solution. Higher concentrations of KOH will result in higher pH values.




