Silver chloride is an inorganic compound with the chemical formula AgCl. It represents the composition of the compound, where Ag represents the chemical symbol for silver, and Cl represents the chemical symbol for chlorine.
It is a white crystalline solid that is highly insoluble in water. Silver chloride is often found naturally as the mineral chlorargyrite or “horn silver.”
Silver chloride is widely used in various applications due to its unique properties.
Structural Formula of Silver Chloride
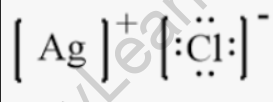
It’s important to note that silver chloride is an ionic compound, meaning it consists of positively charged silver ions (Ag⁺) and negatively charged chloride ions (Cl⁻). The structural formula provided here represents the arrangement of the ions in the crystal lattice, with silver cations and chloride anions arranged in a regular pattern.
Uses of Silver Chloride
- Photography: Silver chloride has been historically used in traditional photography as a light-sensitive material. It was a key component in black and white photographic papers and films, where it reacted with light to create a latent image that could be developed.
- Electrochemistry: Silver chloride is widely used in electrochemical applications, including as an electrode material. It exhibits good electrical conductivity and stability, making it suitable for use in batteries, sensors, and electrochemical cells.
- Medicinal and healthcare applications: Silver chloride has antimicrobial properties and has been used in medical and healthcare settings. It can be incorporated into wound dressings and bandages to help prevent infections and promote wound healing.
- Laboratory reagent: Silver chloride is used as a laboratory reagent in various chemical analyses and experiments. It is often employed in analytical chemistry for precipitation reactions and as a source of silver ions.
- Manufacturing of silver-based products: Silver chloride can serve as a precursor for the production of other silver compounds or materials. It can be used as a starting material in the synthesis of silver nanoparticles, silver salts, and other silver-based products.
Physical properties of Silver Chloride Formula
- Appearance: Silver chloride is a white crystalline solid. It is often observed as a powder or in the form of small crystals.
- Melting Point: The melting point of silver chloride is relatively high, around 455 degrees Celsius (851 degrees Fahrenheit). At this temperature, solid silver chloride transitions to a liquid state.
- Solubility: Silver chloride is sparingly soluble in water. It has low solubility, with a solubility product (Ksp) value of approximately 1.77 x 10-10 at room temperature. This means that only a small amount of silver chloride can dissolve in water.
- Density: The density of silver chloride is around 5.56 grams per cubic centimeter (g/cm³). It is a relatively dense compound.
- Crystal Structure: Silver chloride crystallizes in a face-centered cubic (FCC) crystal lattice structure. In this arrangement, silver ions (Ag⁺) and chloride ions (Cl⁻) are arranged in a repeating pattern throughout the crystal.
- Optical Properties: Silver chloride is a colorless compound, but it can develop a slight yellowish tint upon exposure to light. It is also known for its sensitivity to light, undergoing a photochemical reaction when exposed to certain wavelengths of light.
- Electrical Conductivity: Silver chloride is a poor conductor of electricity in its solid state. However, when it is exposed to light, it can undergo a photochemical reaction that increases its electrical conductivity.
- Stability: Silver chloride is relatively stable under normal conditions. It is not easily decomposed or chemically reactive, although it can be reduced back to silver metal in the presence of reducing agents or upon exposure to intense light.
Chemical Properties of Silver Chloride Formula
- Stability: Silver chloride is relatively stable under normal conditions. It is insoluble in water and does not readily decompose or react with most common substances. However, it can slowly react with atmospheric moisture, forming a thin layer of silver oxide (Ag2O) on its surface.
- Solubility: Although silver chloride is generally considered insoluble in water, it is slightly soluble in aqueous solutions containing certain complexing agents such as ammonia (NH3) or thiosulfate ions (S2O3²⁻). In the presence of these ligands, the formation of soluble complex ions can enhance the solubility of silver chloride.
- Photochemical Reactivity: One of the notable chemical properties of silver chloride is its sensitivity to light. When exposed to certain wavelengths of light, silver chloride undergoes a photochemical reaction known as photo reduction. It is reduced back to silver metal (Ag) by the absorption of photons, typically in the ultraviolet or blue regions of the electromagnetic spectrum.
- Precipitation Reactions: Silver chloride can be formed as a precipitate in a chemical reaction when a soluble silver salt, such as silver nitrate (AgNO3), is mixed with a soluble chloride salt, such as sodium chloride (NaCl). The reaction produces insoluble silver chloride as a white precipitate.
- Redox Reactions: Silver chloride can participate in redox reactions. For example, it can be reduced to silver metal (Ag) by strong reducing agents, such as elemental hydrogen (H2) or metallic zinc (Zn), in appropriate conditions.
- Complex Formation: Silver chloride can form complexes with various ligands, including ammonia (NH3), cyanide (CN⁻), and thiosulfate (S2O3²⁻). These complexes can alter the properties and solubility of silver chloride in solution.
Conclusion
In conclusion, the formula for silver chloride (AgCl) represents a compound composed of silver (Ag) cations and chloride (Cl) anions. Silver chloride is a white crystalline solid that is sparingly soluble in water. It is widely used in various applications, including photography, silver-based electrical contacts, and as a precursor for the synthesis of other silver compounds. Its formula reflects the specific combination of elements that make up this compound, which determines its properties and applications.
Solved Examples on Silver Chloride Formula
Example 1: Calculating the Mass of Silver Chloride Formed
Question: What mass of silver chloride is formed when 5.00 grams of silver nitrate (AgNO3) reacts with excess sodium chloride (NaCl)?
Solution: The balanced chemical equation for the reaction between silver nitrate and sodium chloride is:
AgNO3 + NaCl → AgCl + NaNO3
From the equation, we can see that the molar ratio between AgNO3 and AgCl is 1:1. This means that one mole of AgNO3 reacts to form one mole of AgCl.
To calculate the mass of AgCl formed, we need to determine the number of moles of AgNO3 in 5.00 grams of AgNO3. The molar mass of AgNO3 is 169.87 g/mol.
Number of moles of AgNO3 = mass of AgNO3/ molar mass of AgNO3
= 5.00 g / 169.87 g/mol = 0.0294 mol
Since the molar ratio between AgNO3 and AgCl is 1:1, the number of moles of AgCl formed will also be 0.0294 mol.
Now, we can calculate the mass of AgCl using its molar mass. The molar mass of AgCl is 143.32 g/mol.
Mass of AgCl = number of moles of AgCl × molar mass of AgCl
= 0.0294 mol × 143.32 g/mol = 4.20 grams
Therefore, 4.20 grams of silver chloride will be formed when 5.00 grams of silver nitrate reacts with excess sodium chloride.
Example 2: Determining the Limiting Reagent
Question: If 10.0 grams of silver chloride (AgCl) is reacted with 15.0 grams of sodium nitrate (NaNO3), which is the limiting reagent, and how much excess reagent is left?
Solution: To determine the limiting reagent, we need to compare the number of moles of AgCl and NaNO3 and see which one is present in a lesser amount. The balanced chemical equation for the reaction between AgCl and NaNO3 is:
AgCl + NaNO3→ AgNO3 + NaCl
First, we calculate the number of moles of AgCl:
Number of moles of AgCl = mass of AgCl / molar mass of AgCl = 10.0 g / 143.32 g/mol
= 0.0697 mol
Next, we calculate the number of moles of NaNO3:
Number of moles of NaNO3 = mass of NaNO3/ molar mass of NaNO3
= 15.0 g / 85.00 g/mol = 0.176 mol
From the balanced equation, the molar ratio between AgCl and NaNO3 is 1:1. Therefore, the stoichiometric ratio is 1 mole of AgCl to 1 mole of NaNO3.
Comparing the number of moles, we can see that 0.0697 mol of AgCl is less than 0.176 mol of NaNO3. This means that AgCl is the limiting reagent.
To determine the excess reagent, we subtract the moles of the limiting reagent from the moles of the other reagent.
Excess NaNO3 = moles of NaNO3 – moles of AgCl = 0.176 mol – 0.0697 mol = 0.1063 mol
Frequently Asked Questions on Silver Chloride Formula
1: Why the formula of silver chloride is AgCl?
Answer: The formula AgCl is used to represent silver chloride, indicating the presence of one silver ion (Ag⁺) and one chloride ion (Cl⁻) in each unit of the compound.
2: Which acid is used to prepare silver chloride?
Answer: Silver chloride (AgCl) is typically prepared using hydrochloric acid (HCl). Here’s a general procedure for preparing silver chloride using hydrochloric acid:
- Start with a soluble silver salt, such as silver nitrate (AgNO3), in aqueous solution.
- Add a solution of hydrochloric acid (HCl) to the silver salt solution. The hydrochloric acid reacts with the silver ions to form silver chloride as a precipitate.
The reaction can be represented by the following equation:
AgNO3 (aq) + HCl (aq) → AgCl (s) + HNO3 (aq)
In this reaction, the silver nitrate (AgNO3) reacts with hydrochloric acid (HCl) to produce silver chloride (AgCl) as a solid precipitate, along with nitric acid (HNO3) as a byproduct.
The formed silver chloride precipitate can then be separated by filtration and washed to remove any impurities.
3: What is the use of silver chloride ?
Answer: Silver chloride (AgCl) has several important uses. One of its primary applications is in traditional black and white photography, where it is used in photographic films and papers as a light-sensitive material. It undergoes a photochemical reaction when exposed to light, forming silver metal and creating the desired image. Silver chloride also finds use in analytical chemistry for detecting and quantifying chloride ions through precipitation reactions. Additionally, it has antimicrobial properties and is utilized in certain medical and dental applications, such as wound dressings and creams. Overall, silver chloride’s uses span photography, analytical chemistry, and medical applications due to its unique properties.
4: What are some interesting facts about silver chloride?
Answer: Here are some interesting facts about silver chloride (AgCl):
- Light Sensitivity: Silver chloride is highly sensitive to light, particularly in the blue and ultraviolet regions of the electromagnetic spectrum. This property makes it valuable in photography as a light-sensitive material.
- Color Changes: While silver chloride is typically white, it can develop a yellowish or grayish tint upon prolonged exposure to light or certain environmental conditions. This color change is attributed to the formation of silver oxide (Ag2O) on the surface of the compound.
- Photochemistry: When exposed to light, silver chloride undergoes a photochemical reaction known as photo reduction. It is reduced back to metallic silver, which leads to changes in its properties, including electrical conductivity.
5: Which colour does silver chloride turn in sunlight?
Answer: Silver chloride (AgCl) turns dark gray or black when exposed to sunlight. This color change is due to the photochemical reaction that occurs within the compound. When silver chloride is exposed to light, it undergoes a reduction reaction, resulting in the formation of metallic silver (Ag) particles. The accumulation of these silver particles gives the silver chloride a dark appearance. This property of turning dark in sunlight is one of the distinctive characteristics of silver chloride.
6: Is it AgCl or AgCl2?
Answer: The chemical formula for silver chloride is AgCl, not AgCl2. Silver chloride is a compound composed of one silver ion (Ag+) and one chloride ion (Cl–). The subscript “2” in a chemical formula typically indicates the presence of two ions of that element, but in the case of silver chloride, only one chloride ion is combined with one silver ion. Therefore, the correct formula for silver chloride is AgCl.
7: What is the valency of AgCl?
Answer: In the compound silver chloride (AgCl), the valency of silver (Ag) is +1, and the valency of chloride (Cl) is -1. The valency indicates the combining capacity of an element or ion, representing the number of electrons it gains, loses, or shares when forming chemical bonds. In AgCl, the silver ion (Ag+) has a positive valency of +1, indicating that it donates one electron, while the chloride ion (Cl–) has a negative valency of -1, indicating that it accepts one electron.
8: What is the colour of AgCl precipitate?
Answer: The precipitate of silver chloride (AgCl) is typically white. Silver chloride is an insoluble compound, and when it forms as a precipitate in a chemical reaction, it appears as a white solid. The white color is due to the scattering of light by the fine particles of AgCl in the solution.


