Table of Contents
Introduction Sodium Nitrite Formula
Sodium nitrite, with the formula NaNO2, is a white crystalline powder that is highly soluble in water. It is commonly used as a food preservative, particularly in cured meats, to inhibit bacterial growth and prevent spoilage. Sodium nitrite also has medical applications, such as the treatment of cyanide poisoning and methemoglobinemia. It is used in industrial processes, including dye manufacturing and metal treatment. Sodium nitrite is an oxidizing agent and can release toxic nitrogen dioxide gas at high temperatures. It should be handled with care due to its reactivity and potential toxicity.
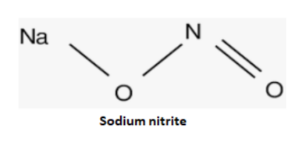
The formula for sodium nitrite is NaNO2. Here are the additional details about sodium nitrite:
Structure of Sodium Nitrite
Sodium nitrite consists of one sodium ion (Na+) and one nitrite ion (NO2-) in its structure. The nitrite ion consists of one nitrogen atom bonded to two oxygen atoms.
Physical properties of Sodium Nitrite
- Appearance: Sodium nitrite is a white or slightly yellowish crystalline powder.
- Solubility: It is highly soluble in water.
- Melting point: Sodium nitrite has a melting point of 271°C.
- Density: The density of sodium nitrite is approximately 2.17 g/cm3.
Chemical properties of Sodium Nitrite
- Oxidizing agent: Sodium nitrite is an oxidizing agent, meaning it can donate oxygen or accept electrons from other substances in chemical reactions.
- Decomposition: At high temperatures, sodium nitrite can decompose to release toxic nitrogen dioxide gas (NO2).
- Reaction with acids: Sodium nitrite reacts with acids to produce nitrous acid (HNO2), which can further decompose to release nitrogen dioxide.
Uses of Sodium Nitrite
- Food preservative: Sodium nitrite is commonly used as a food preservative, particularly in cured meat products. It helps inhibit the growth of bacteria and prevents spoilage.
- Medicinal applications: Sodium nitrite is used in medicine for the treatment of certain medical conditions such as cyanide poisoning and methemoglobinemia.
- Industrial applications: It is utilized in various industrial processes, including dye manufacturing, metal treatment, and as a reducing agent in chemical reactions.
It is important to note that sodium nitrite should be handled with care due to its potential toxicity and reactivity.
Solved examples on Sodium Nitrite (NaNO2):
Example 1: A 50.0 mL solution contains 0.25 moles of sodium nitrite (NaNO2). What is the molarity of the solution?
Solution:
Molarity (M) is defined as moles of solute divided by volume of solution in liters.
Given:
Moles of sodium nitrite (NaNO2) = 0.25 moles
Volume of solution = 50.0 mL = 0.050 L
Molarity (M) = Moles of solute / Volume of solution
M = 0.25 moles / 0.050 L
M = 5.0 M
Therefore, the molarity of the solution is 5.0 M.
Also Check: Zinc Nitrate Formula
Example 2: A reaction between sodium nitrite (NaNO2) and hydrochloric acid (HCl) produces sodium chloride (NaCl), nitrogen dioxide gas (NO2), and water (H2O). How many moles of sodium nitrite are required to react with 1.5 moles of hydrochloric acid?
Solution:
The balanced chemical equation for the reaction is
2 NaNO2 + 4 HCl -> 2 NaCl + 2 NO2 + 2 H2O
According to the stoichiometry of the equation, 2 moles of sodium nitrite react with 4 moles of hydrochloric acid.
Given:
Moles of hydrochloric acid (HCl) = 1.5 moles
Using the stoichiometry of the reaction, the number of moles of sodium nitrite required is:
Moles of sodium nitrite = (1.5 moles HCl / 4 moles HCl) * 2 moles NaNO2
Moles of sodium nitrite = 0.75 moles NaNO2
Therefore, 0.75 moles of sodium nitrite are required to react with 1.5 moles of hydrochloric acid.
Frequently Asked Question on Sodium nitrate
What is sodium nitrite used for?
Sodium nitrite is used for various purposes, including: Preserving and curing meats: It's used as a preservative and to give processed meats (like bacon, sausages, and ham) their characteristic pink color and flavor. Inhibiting bacterial growth: Sodium nitrite helps prevent the growth of harmful bacteria like Clostridium botulinum in processed meats.
Is sodium nitrite safe to eat?
In small amounts, sodium nitrite is generally considered safe to consume. However, excessive consumption can be harmful, and there are concerns about the formation of nitrosamines, which are potentially carcinogenic compounds, when sodium nitrite reacts with certain components in food during cooking or processing. Therefore, its use in food is regulated, and specific limits are set to ensure safety.
Is sodium nitrite the same as sodium nitrate?
No, sodium nitrite (NaNO2) and sodium nitrate (NaNO3) are not the same. They are different chemical compounds with distinct properties. Sodium nitrate is often used as a preservative in some processed meats, while sodium nitrite is used in conjunction with sodium nitrate or on its own.
What is the density of sodium nitrite in g/mL?
The density of sodium nitrite at room temperature (approximately 20°C or 68°F) is about 2.168 grams per milliliter (g/mL).
What is the density of sodium nitrate?
The density of sodium nitrate at room temperature is approximately 2.261 grams per milliliter (g/mL).
Why is sodium nitrite banned?
Sodium nitrite is not generally banned, but its use in food is heavily regulated due to concerns about its potential to form nitrosamines, which are associated with cancer risk. Regulations are in place to limit its use in processed meats and ensure it is used within safe levels.
Is sodium nitrate OK to eat?
Sodium nitrate is considered safe to consume when used in accordance with regulatory guidelines and within established limits. It is commonly used as a food preservative in some processed meats.
How toxic is sodium nitrate to humans?
Sodium nitrate itself is not highly toxic to humans when consumed in small amounts through food. However, excessive intake of nitrate or the formation of nitrosamines from nitrate and nitrite in the digestive tract can be harmful and is associated with health risks, particularly an increased risk of certain cancers. This is why regulatory agencies set limits on their use in food products to minimize health risks.








