Table of Contents
Specific gravity is a measurement that compares the density of a substance to the density of a reference substance, usually water. It provides a relative indication of how dense or light a substance is compared to water. The specific gravity is calculated by dividing the density of the substance by the density of water. It is a dimensionless quantity and does not have any units. Specific gravity is commonly used in various industries and applications, such as determining the purity of liquids, evaluating the buoyancy of objects, and assessing the concentration of solutions. It plays a crucial role in understanding the physical properties and behavior of different substances.
Definition and Formula of Specific Gravity:
Specific gravity or Relative density is the ratio of the density (mass of a unit volume) of a substance to the density of the given reference material. Generally, the reference material is water.
The relative density/specific gravity formula is:
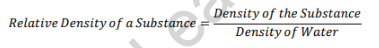
Unit of specific gravity:
Specific gravity is a dimensionless quantity and does not have any units. It is simply a ratio of densities, so it is expressed as a number without any accompanying units
Characteristics of Specific Gravity:
The characteristics of specific gravity are:
- It has no units as it is a ratio of two similar quantities.
- It is constant only under given conditions. It is pressure and temperature-dependent. The same substance can have different relative densities under different pressures or temperatures.
- It can never be zero.
Applications of Specific Gravity:
The relative density or specific gravity helps us to find if,
- The object is denser than water.
- The object will float or sink in water. If the relative density of a substance is greater than one, then it will sink. And if it is lesser than one, it will float.

Relative Density or Specific Gravity: Examples
Let us try to find out the relative density of silver and ice using the formula of relative density.
Relative Density of Silver
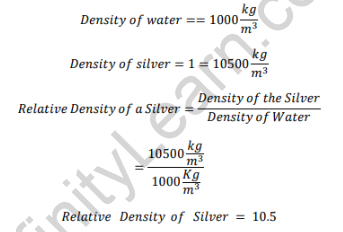
Here, silver is approximately 10.5 times denser than water under the given conditions. Since its relative density is greater than 1, silver will sink in water.
Relative Density of Ice
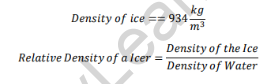
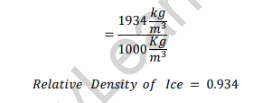
As the relative density of ice is less than one, it will float in water.
Solved Examples on Specific Gravity Formula:
Example 1: The density of a substance is 2.5 g/cm³. Calculate its specific gravity relative to water.
Solution:
Given:
Density of the substance = 2.5 g/cm³
Density of water = 1 g/cm³
Using the formula for specific gravity:
Specific Gravity = Density of Substance / Density of Water
Substituting the given values:
Specific Gravity = 2.5 g/cm³ / 1 g/cm³
Specific Gravity = 2.5
Therefore, the specific gravity of the substance relative to water is 2.5.
Example 2: A liquid has a specific gravity of 0.8. Determine its density in kg/m³ if the density of water is 1000 kg/m³.
Solution:
Given:
Specific Gravity = 0.8
Density of water = 1000 kg/m³
Using the formula for specific gravity: Specific Gravity = Density of Substance / Density of Water
Rearranging the formula to solve for the density of the substance:
The density of Substance = Specific Gravity x Density of Water
Substituting the given values:
Density of Substance = 0.8 x 1000 kg/m³
Density of Substance = 800 kg/m³
Therefore, the density of the liquid is 800 kg/m³.
Example 3: A solid has a specific gravity of 3.75. Find its density in g/cm³ if the density of water is 1 g/cm³.
Solution:
Given:
Specific Gravity = 3.75
Density of water = 1 g/cm³
Using the formula for specific gravity: Specific Gravity = Density of Substance / Density of Water
Rearranging the formula to solve for the density of the substance: Density of Substance = Specific Gravity x Density of Water
Substituting the given values:
Density of Substance = 3.75 x 1 g/cm³
Density of Substance = 3.75 g/cm³
Therefore, the density of the solid is 3.75 g/cm³.
FAQ’s on Specific Gravity
What is specific gravity?
Specific gravity is a measure of the density of a substance relative to the density of a reference substance, typically water. It provides a comparison of the heaviness or density of a substance compared to water.
What is the unit of specific gravity?
Specific gravity is a dimensionless quantity since it is a ratio of two densities. It does not have any units associated with it.
What factors affect specific gravity?
Specific gravity is influenced by two main factors: the density of the substance being compared, and the reference substance used. The density of the substance is determined by its mass and volume, while the reference substance is typically water. Therefore, any changes in the mass or volume of the substance or variations in the density of the reference substance can impact the specific gravity value.
How to calculate specific gravity?
The specific gravity is calculated by dividing the density of the substance by the density of the reference substance.
What is the specific gravity of water?
The specific gravity of pure water is approximately 1. It is often used as the reference substance with a specific gravity of 1 for comparison with other substances to determine their relative density or buoyancy.
What are the properties of specific gravity?
The specific gravity of a substance is a dimensionless quantity that represents the ratio of its density to the density of a reference substance. It provides information about the relative heaviness or lightness of a substance compared to the reference substance. Specific gravity is commonly used in various applications, such as determining the purity of materials, calculating buoyancy, and characterizing the density of liquids and solids.
How is specific gravity different from density?
Density is an absolute measurement of how much mass is contained in a given volume of a substance. Specific gravity, on the other hand, is a relative measurement that compares the density of a substance to the density of a reference substance.
What is the reference substance for specific gravity?
The reference substance for specific gravity is often water. The density of water is typically used as a standard reference point, with a specific gravity of 1.
How is specific gravity used in practical applications?
Specific gravity is used in various industries and applications. It is utilized in determining the purity of substances, assessing the buoyancy and floatability of materials, evaluating the concentration of solutions, and understanding the behavior of substances in different environments.
How does specific gravity relate to buoyancy?
Specific gravity is closely related to buoyancy. If the specific gravity of a substance is less than 1, it will float in water, indicating it is less dense than water. If the specific gravity is greater than 1, the substance will sink in water, indicating it is denser than water.
Can specific gravity be greater than 1?
Yes, specific gravity can be greater than 1. A specific gravity greater than 1 indicates that the substance is denser than the reference substance (e.g., water).
Can specific gravity be negative?
No, specific gravity cannot be negative. It is always a positive value or zero. Negative values do not make physical sense in the context of specific gravity.
Why is it called specific gravity?
Specific gravity is called so because it quantifies the specific or relative density of a substance compared to a reference substance. It provides a measure of how much denser or lighter a substance is compared to another substance of known density. The term specific in specific gravity emphasizes the comparison to a specific reference material, often water, which has a defined density. By comparing the density of a substance to the density of water, specific gravity allows for a standardized measurement of density that is independent of the units used.








