Hydrocarbons Class 11 Notes Chemistry Chapter 13
• Hydrocarbon: A compound of carbon and hydrogen is known as a hydrocarbon.
• Saturated Hydrocarbon: A hydrocarbon is said to be saturated if it contains only C—C single bonds. For example Ethane CH3—CH3
• Unsaturated Hydrocarbon

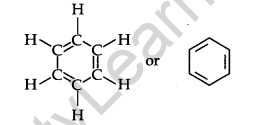
• Aromatic Hydrocarbon: Benzene and its derivatives are called aromatic compounds.
Example:
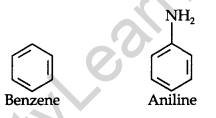
• Alicyclic Compounds: Cyclic compounds which consist only of carbon atoms are called alicyclic or carbocyclic compounds.

• Heterocyclic Compounds: Cyclic compounds in which the ring atoms are of carbon and some other element (For example, N, S, or O) are called heterocyclic compounds.

• Alkanes: Alkanes are the simplest organic compounds made of carbon and hydrogen only.
They have the general formula CnHC2n+2 (where n = 1, 2, 3, etc.)
The carbon atoms in their molecules are bonded to each other by single covalent bonds. Since the carbon skeleton of alkanes is fully saturated’ with hydrogens, they are also called saturated hydrocarbons. Alkanes contain strong C —C and C —H bonds. Therefore, this class of hydrocarbons are relatively chemically inert. Hence they are sometimes referred to as paraffin (Latin parum affinis = little affinity). The first three members of this class can be represented as
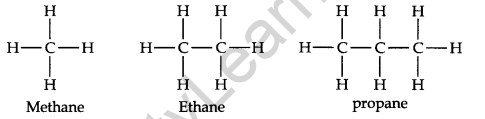
Structure:
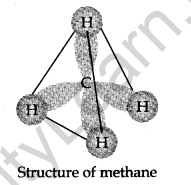
In methane, carbon forms single bonds with four hydrogen atoms. All H—G—H bond angles are 109.5°. Methane has a tetrahedral structure. C—C and C—H bonds are formed by head-on overlapping of sp3 hybrid orbitals of carbon and Are orbitals of hydrogen atoms.
• Nomenclature Guidelines
Use the following step-by-step procedure to write the IUPAC names from the structural formulas. Consider the following structural formula:
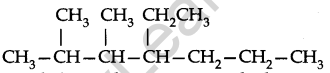
Step 1. Identify the longest chain: In the given example, the longest chain has seven carbons. The seven carbon chain is heptane.
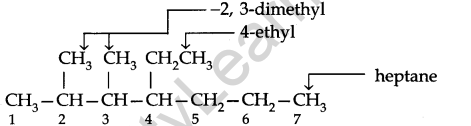
Step 2. Number the chain: The chain is numbered from left to right. This gives the lowest numbers to the attached alkyl group.
Step 3. Identify the alkyl group: There are two methyl groups at C-2 and C-3, there is one ethyl group of C-4.
Step 4. Write the IUPAC name: In this case, the IUPAC name is 4-Ethyl-2,3-dimethyl heptane. Always keep in mind (a) Numbers are separated from each other by commas. (b) Numbers are separated from names by hyphens, (c) Prefixes di, tri is not taken into account in alphabetising substituent names.
• Newman Projections
In this projection, the molecule is viewed at the C—C bond head-on.
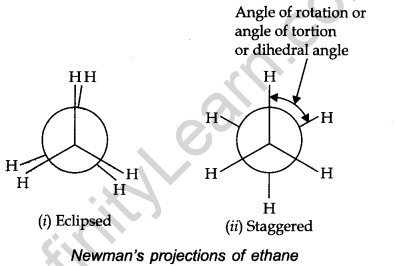
• Relative Stability of Conformations
In the staggered form of ethane, there are maximum repulsive forces, minimum energy and maximum stability of the molecule. On the other hand, when the staggering form changes in the eclipsed form the electron clouds of the carbon-hydrogen bonds come closer to each other increasing electron cloud repulsions, molecule have to possess more energy and thus has lower stability.
Torsional Angle: The magnitude of torsional strain depends upon the angle of rotation about the C—C bond. This angle is also called a dihedral angle or torsional angle.
• Alkenes
Alkenes are hydrocarbons that contain a carbon-carbon double bond (C=C) in their molecule.
They have the general formula
Structure:
Let us consider (H2C=CH2) for illustrating the orbital make-up of alkenes.
In ethylene the carbon atoms are sp2 hybridized- They are attached by a bond and a σ bond.
The bond results from the overlap of two sp2 hybrid orbitals. The π bond is formed from the overlap of the unhybridized p-orbitals. Ethylene is a planar molecule.
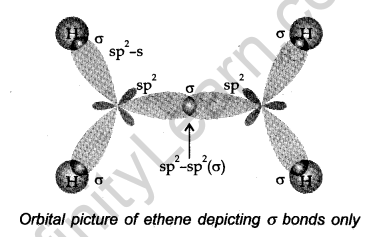
Points to be noted
(i) The carbon-carbon double bond in alkenes is made up of one σ and one π-bond.
(ii) Alkenes are more reactive than Alkanes. This is due to the availability of n electrons.
• Nomenclature
In IUPAC system
(i) The name of the hydrocarbon is based on the parent alkene having the longest ‘ carbon chain of which double bond is apart.
(ii) This chain is numbered from the end near the double bond and its position is indicated by the number of the carbon atom not which the double bond originates,
(iii) The name of the parent alkene with the position number of the double bond is written first and then the names of other substituents are prefixed to it.
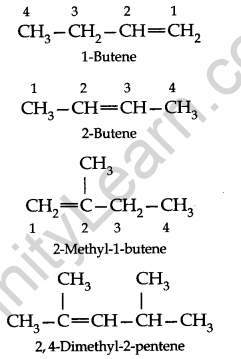
(iv) When there are two or three double bonds in a molecule, the ending-one of the corresponding alkane is replaced by-a diene to get the name.

• Isomerism
Structural Isomerism: Ethene and propene have no structural isomers, but there are three structures of butenes.
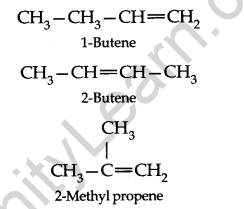
Of these, two are straight-chain structures with the difference being in the position of the double bond in the molecules.
These are position isomers and the third structure is a branched-chain isomer.
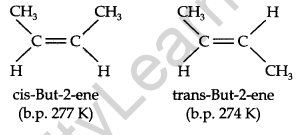
Geometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.
This presentation of rotation about the carbon-carbon double bond gives rise to the phenomenon of geometrical isomerism. An alkene having a formula RCH=CHR can have two stereoisomers, depending upon whether the two alkyl groups are on the same or opposite sides of the double bond. If they are on the same side, then it is called cis-isomer. If they are on opposite sides, then it is called trans-isomer.
Due to the different arrangements of atoms or groups in space, these isomers differ in their properties like melting point, boiling point, dipole moment, solubility, etc.
• Alkynes
Alkynes are characterised by the presence of a triple bond in the molecule.
Their general formula is CnH2n-2.
The first and the most important member of this series of hydrocarbons is acetylene, HC=CH, and hence they are also called the Acetylenes.
Structure: Let us consider ethyne (HC=CH) for illustrating the orbital make-up of ethyne. In ethyne, the carbon atoms are sp hybridized. They are attached by a σ-bond and two π-bonds.
The σ -bond results from the overlap of two sp hybrid orbitals. The π bonds are formed from the separate overlap of the two p-orbitals from the two adjacent carbon atoms.
The other sp hybrid orbital of each carbon atom forms a σ bond with another carbon or hydrogen atom. Ethyne is a linear molecule.
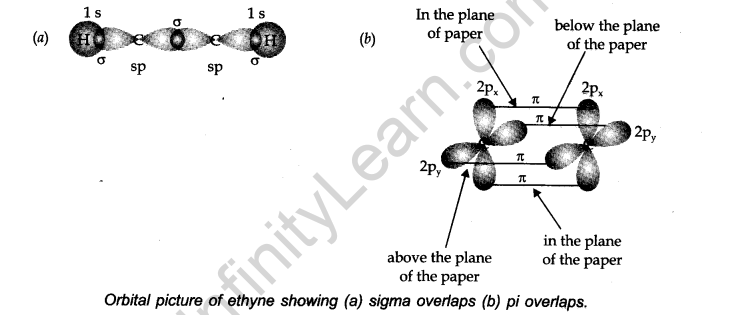
Points to be noted:
(i) The carbon-carbon triple bond in alkynes is made up of one σ and two π bonds.
(ii) Like alkenes, alkynes undergo an addition reaction. These reactions are due to the availability of more exposed π electrons.
• Nomenclature
IUPAC System: The IUPAC names of alkynes are obtained by dropping the ending-ane of the parent alkane and adding the suffix-ya. The carbon chain including the triple bond is – numbered from the end nearest this bond. The position of the triple bond is indicated by prefixing the number of carbon preceding it to the name of the alkyne.
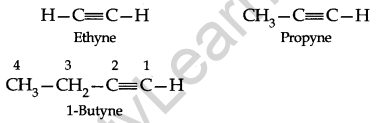
Preparation:
From calcium carbide: Ethyne is prepared by treating calcium carbide with water. Calcium carbide is prepared as follows:
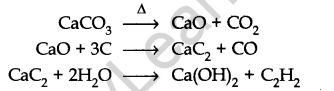
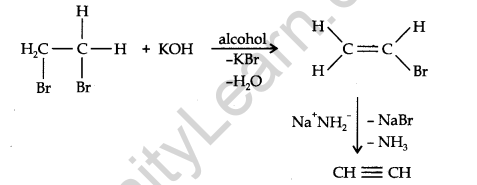
From vicinal dihalides: When reacted with vicinal dihalides, alcoholic potassium hydroxide undergo dehydrohalogenation. One molecule of hydrogen halide is eliminated to form alkenyl halide which on treatment with sodamide gives alkyne.
• Aromatic Hydrocarbons
These hydrocarbons are also known as ‘arenes’. Most of such compounds were found to contain a benzene ring.
Aromatic compounds containing benzene rings are known as benzenoids and those not containing a benzene ring are known as non-benzenoids. Some examples of arenes are given below.
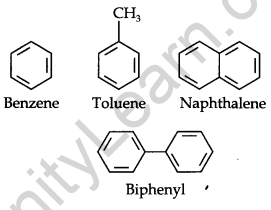
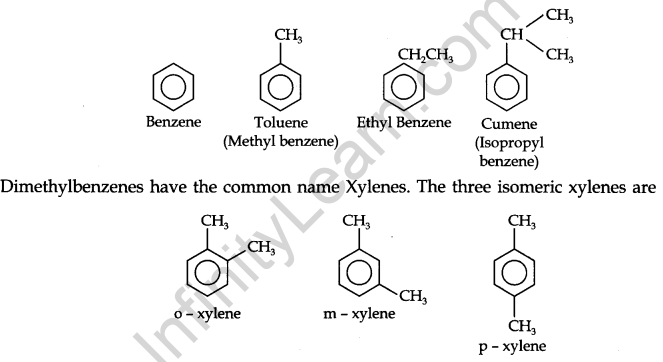
Nomenclature and Isomerism: Benzene and its homologous are generally called by their common names which are accepted by the IUPAC system. The homologous benzene having a single alkyl group is named Alkyl benzenes.
Structure of Benzene: By elemental analysis, it is found that the molecular formula of benzene is C6H6. This indicates that benzene is a highly unsaturated compound. In 1865, Kekule gave the cyclic planar structure of benzene with six carbons with alternate double and single bonds.
The Kekule structure indicates the possibility of two isomeric 1,2-bromobenzene. In one of the isomers, the bromine atoms are attached to the doubly bonded carbon atoms whereas in the other they are attached to the singly bonded carbon.
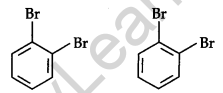
Only one ortho-bromobenzene could be prepared.
To overcome this problem Kekule suggested that benzene was a mixture of two forms.
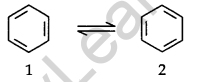
Failure of Kekule’s structure: Kekule structure of benzene failed to explain the unique stability and its preference to substitution reaction than addition reactions.
Resonance Structure of Benzene: The phenomenon in which two or more structures can be written for a substance that involves identical positions of atoms is called resonance. In benzene’s, Kekule’s structures (1) and (2) represent the resonance structures. The actual structure – of the molecule is represented by a hybrid of these two structures.
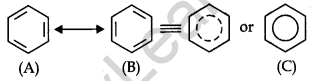
Orbital structure of benzene: All six carbon atoms in benzene are sp2 hybridized. The sp2 hybrid orbitals overlap with each other and with s orbitals of the six hydrogen atoms forming C—C and C—H σ-bonds.
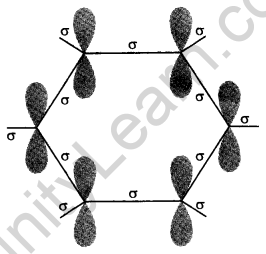
X-ray diffraction data indicate that benzene is a planar molecule. The data indicate that all the six C—C bond lengths are of the same order (139 pm) which is intermediate between (C—C) single bond (154 pm) and C—C double bond (133 pm). Thus the presence of pure double bond in benzene gives the idea of the reluctance of benzene to show addition reaction under normal conditions. The is, It explains the unusual behaviour of benzene.
Aromaticity: It is a property of the sp2 hybridized planar rings in which the p orbitals allow cyclic delocalization of π electrons.
Conditions for Aromaticity:
(i) An aromatic compound is cyclic and planar.
(ii) Each atom in an aromatic ring has a p orbital. These p orbitals must be parallel so that a continuous overlap is possible around the ring.
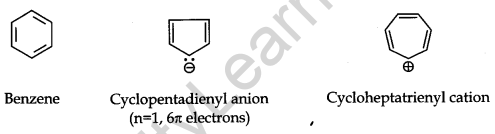
(iii) The cyclic π molecular orbital (electron cloud) formed by the overlap of p orbitals must contain (4n + 2) π electrons. Where n = integer (0, 1, 2, 3, etc.). This is known as the Huckel rule.
Some Examples of Atomic Compounds are given below:
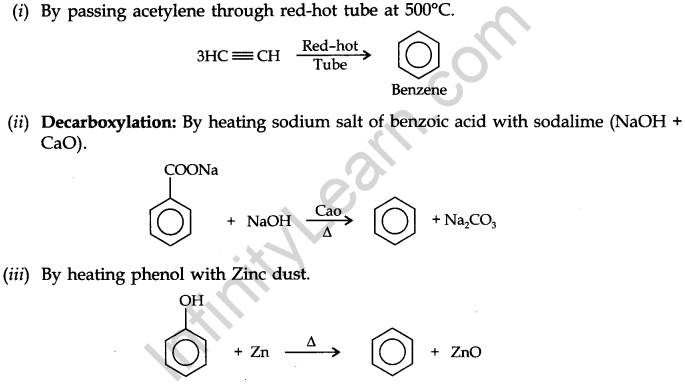
Preparation of Benzene: Benzene is commercially isolated from coal tar. However, some synthetic methods are applied in the laboratory for the preparation of benzene.
Physical Properties of Benzene:
(i) Benzene is a colourless liquid.
(ii) It is’ insoluble in water. It is soluble in alcohol, ether, chloroform etc.
(iii) Benzene itself is a good solvent for many organic and inorganic substances e.g., fat, resins, sulphur and iodine.
(iv) It bums with a luminous, sooty flame in contrast to alkanes and alkenes which usually bum with a bluish flame.
Chemical Properties:
Benzene undergoes the following types of chemical reactions.
(i) Electrophillic Substitution Reaction
(ii) Addition Reaction
Electrophillic Substitution Reactions:
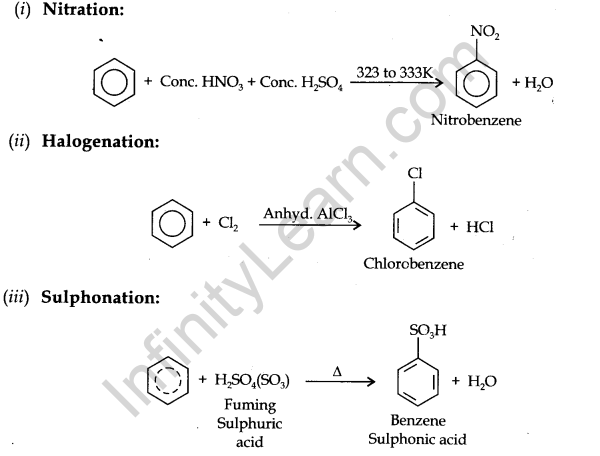
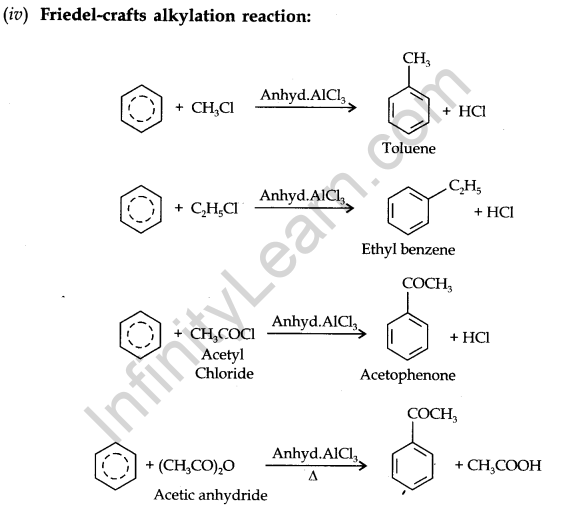
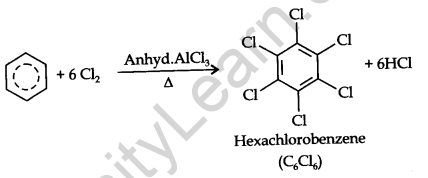
Benzene on treatment with an excess of chlorine in the presence of anhydrous AlCl3 can be chlorinated to hexachlorobenzene (C6Cl6)
Mechanism of electrophilic substitution reactions:
All electrophilic substitution reactions follow the same three-step mechanism.
Step 1. Formation of an electrophile:
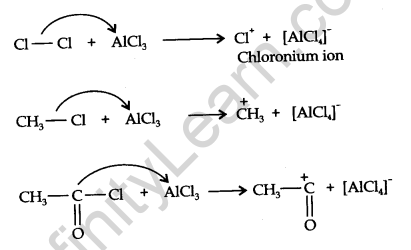
Step 2. The electrophile attacks the aromatic ring to form a carbonium ion.
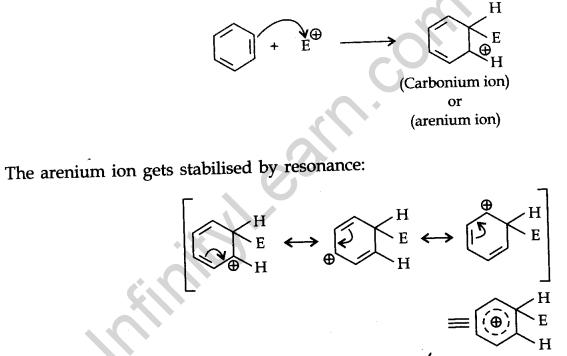
Step 3. Loss of proton gives the substitution product.
Activating groups: This group activates the benzene ring for the attack by an electrophile. Example, —OH; —NH2, —NHR, —NHCOCH3, —OCH3 —CH3 —C2H5 etc.
Deactivating groups: Due to deactivating group because of the strong —I effect, overall electron density on benzene ring decreases. It makes further substitution difficult.
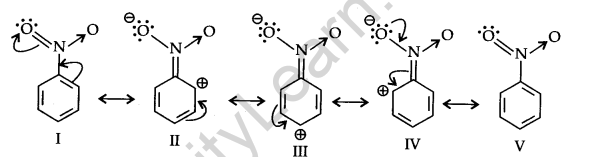
Metadirecting group: The groups which direct the incoming group to meta position are called meta directing groups. Some examples of meta directing groups are —N02, —CN, —CHO, —COR, —COOH, —COOR, -S03H etc.
Let us consider the example of the nitro group: Since the Nitro group due to its strong -I effect reduces the electron density in the benzene ring. Nitrobenzene is a resonance hybrid of the following structures.
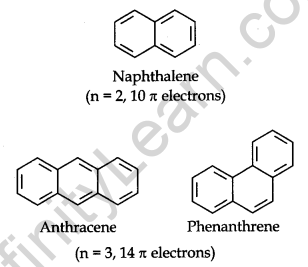
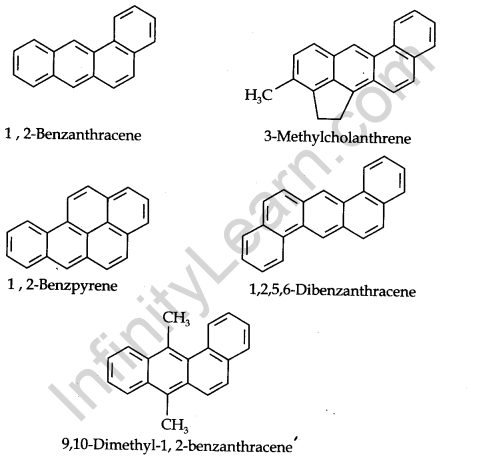
Carcinogenicity and Toxicity: Some polynuclear hydrocarbons containing more than two benzene rings fused become toxic and they are having cancer-producing properties. They are formed due to incomplete combustion of some organic materials like tobacco, coal and petroleum, etc.
Some of the carcinogenic hydrocarbons are given below.
• Hydrocarbons: They are compounds of carbon and hydrogen only.
Open Chain saturated compound—Alkane
Unsaturated Compound—Alkenes and Alkynes Aromatic Compound—Benzene and its derivatives Terminal alkynes are weakly acidic.
• Conformation: Spatial arrangements obtained by rotation around sigma bonds.
• Eclipsed Conformation: Less stable because of more repulsion between bond pairs of electrons.
• Staggered: It is more stable since there is less repulsion between bond pairs of electrons.
• Geometrical isomerism: Observed only in compounds containing a double bond.
• Stability of benzene. This is explained based on resonance hybrid.
• Arenes: Take part in an electrophilic substitution reaction.
Aromaticity is determined by Huckle’s rule (4n + 2) rule.]
For more visit Surface Chemistry Class 12 Notes Chemistry Chapter 5




