Uric Acid Formula
Uric acid is an organic compound that plays a crucial role in the metabolism of purine, a component of nucleic acids (DNA and RNA). It is produced as a result of the breakdown of purines in the body, either from dietary sources or from the natural turnover of cells. The chemical formula for uric acid is C₅H₄N₄O₃.
Uric acid is primarily excreted from the body through urine, and its levels in the bloodstream are tightly regulated by various physiological processes. Elevated levels of uric acid can lead to hyperuricemia, which is associated with conditions such as gout, kidney stones, and certain metabolic disorders.
Uric acid has both antioxidant and pro-oxidant properties and can act as a scavenger of free radicals. It also has been suggested to have a potential role as a neuroprotective agent.
Structural Formula of Uric Acid
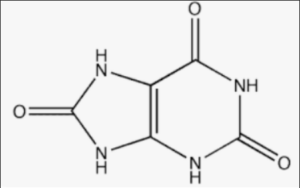
In the structural formula, the atoms are represented by their chemical symbols, and the lines represent the covalent bonds between the atoms. Hydrogen (H), carbon (C), nitrogen (N), and oxygen (O) atoms are present in uric acid.
Uric acid is a heterocyclic compound consisting of a purine ring fused with an imidazole ring. The purine ring is composed of two fused rings, while the imidazole ring is a smaller ring fused to the purine ring.
Uses of Uric Acid
- Antioxidant: Uric acid acts as an antioxidant in the body, helping to neutralize harmful free radicals and protect cells from oxidative damage.
- Nitric Oxide Production: Uric acid can be converted into nitric oxide, a molecule that plays a crucial role in regulating blood vessel dilation, blood pressure, and overall cardiovascular health.
- pH Buffer: Uric acid helps maintain the pH balance of bodily fluids, particularly in the blood, by acting as a buffer and preventing excessive acidity or alkalinity.
- Waste Product Elimination: Uric acid is a waste product of purine metabolism. It is excreted primarily through urine, helping to eliminate excess nitrogen from the body.
Physical properties of Uric Acid Formula
- Molar mass: 143.32 grams/mol
- Appearance: Silver chloride is a white crystalline solid.
- Density: The density of silver chloride is approximately 5.56 grams/cm³.
- Melting point: Silver chloride has a relatively high melting point of around 455 degrees Celsius (851 degrees Fahrenheit).
- Boiling point: It does not have a distinct boiling point since it decomposes before reaching the boiling point.
- Solubility: Silver chloride has low solubility in water, with a solubility product (Ksp) of approximately 1.8 × 10-10 at 25 degrees Celsius. It is more soluble in hot water than in cold water.
- Crystal structure: Silver chloride has a face-centered cubic crystal structure.
- Conductivity: It is a poor conductor of electricity in its solid state. However, when it is exposed to light, it undergoes a photochemical reaction and becomes more conductive due to the formation of silver metal particles.
- Stability: Silver chloride is relatively stable under normal conditions but can be reduced to metallic silver by strong reducing agents or exposure to light.
- Optical properties: It is opaque and exhibits low light transmission due to its crystalline structure.
Chemical Properties of Uric Acid Formula
- Acidic nature: Uric acid is a weak organic acid and can act as a proton donor, making it capable of forming salts and reacting with bases to produce water-soluble urate salts.
- Dissociation: In aqueous solutions, uric acid can partially dissociate to form hydrogen ions (H+) and urate ions (C₅H₃N₄O₃⁻).
- Reaction with bases: Uric acid reacts with bases such as sodium hydroxide (NaOH) to form water-soluble urate salts, such as sodium urate (NaC₅H₃N₄O₃).
- Oxidation: Uric acid is susceptible to oxidation, particularly in the presence of certain enzymes and metal ions. The oxidation of uric acid results in the formation of allantoin, carbon dioxide, and hydrogen peroxide.
- Precipitation: Uric acid can precipitate from urine or other bodily fluids, leading to the formation of uric acid crystals. This can occur when the concentration of uric acid exceeds its solubility limit.
- Reactivity with oxidizing agents: Uric acid can undergo oxidation reactions when exposed to strong oxidizing agents. For example, it can be oxidized to allantoin in the presence of various oxidants.
- Formation of complexes: Uric acid can form complexes with certain metal ions, such as copper, resulting in the formation of insoluble compounds. These complexes are involved in the pathogenesis of certain diseases, including gout.
- Reducing properties: Uric acid exhibits reducing properties and can reduce certain oxidizing agents. It can act as an antioxidant by donating electrons to free radicals and reactive oxygen species.
- Reaction with nitric acid: Uric acid reacts with nitric acid to form a yellow compound called xanthine, which can further react to form other compounds, including alloxan and oxalic acid.
Conclusion
In conclusion, the formula for uric acid is C₅H₄N₄O₃. Uric acid is a naturally occurring compound found in the body and serves various functions. It acts as an antioxidant, helps in the production of nitric oxide, balances pH levels, and aids in the elimination of waste products. However, elevated levels of uric acid can lead to health issues such as gout. Managing uric acid levels is important for maintaining overall health and preventing related conditions.
Solved Examples on Uric Acid Formula
Example 1: Calculate the number of moles of uric acid (C₅H₄N₄O₃) in a 25.0 g sample of uric acid.
Solution: To calculate the number of moles, we need to use the molar mass of uric acid.
The molar mass of uric acid (C₅H₄N₄O₃) can be calculated by summing up the atomic masses of its constituent elements:
C: 5 x 12.01 g/mol = 60.05 g/mol
H: 4 x 1.01 g/mol = 4.04 g/mol
N: 4 x 14.01 g/mol = 56.04 g/mol
O: 3 x 16.00 g/mol = 48.00 g/mol
Molar mass of uric acid = 60.05 + 4.04 + 56.04 + 48.00 = 168.13 g/mol
Now, we can use the formula:
Number of moles = Mass (g) / Molar mass (g/mol)
Number of moles = 25.0 g / 168.13 g/mol ≈ 0.1488 mol (rounded to four decimal places)
Therefore, there are approximately 0.1488 moles of uric acid in a 25.0 g sample.
Example 2: A 0.250 Molar (0.250 M) solution of uric acid (C₅H₄N₄O₃) is prepared by dissolving 26.3 g of uric acid in water. Calculate the volume of water (in liters) needed to prepare this solution.
Solution: To calculate the volume of water, we can use the formula:
Number of moles = Molarity x Volume (in liters)
We already know the molarity of the solution (0.250 M) and the number of moles can be calculated using the formula from Example 1:
Number of moles = Mass (g) / Molar mass (g/mol)
Number of moles = 26.3 g / 168.13 g/mol ≈ 0.1565 mol (rounded to four decimal places)
Now, rearranging the formula for volume:
Volume (in liters) = Number of moles / Molarity
Volume (in liters) = 0.1565 mol / 0.250 M ≈ 0.626 L (rounded to three decimal places)
Therefore, approximately 0.626 liters of water are needed to prepare a 0.250 Molar solution of uric acid.
Frequently Asked Questions on Uric Acid Formula
1: What is the general formula for uric acid?
Answer: The general chemical formula for uric acid is C₅H₄N₄O₃. This formula represents the overall molecular composition of uric acid, where C represents carbon, H represents hydrogen, N represents nitrogen, and O represents oxygen. The subscript numbers indicate the number of atoms of each element in one molecule of uric acid.
2: Which method is most commonly used for uric acid?
Answer: The most commonly used method for the determination of uric acid is the enzymatic method. In this method, the enzyme uricase is used to catalyze the oxidation of uric acid to produce allantoin, carbon dioxide, and hydrogen peroxide. The generated hydrogen peroxide is then measured using a colorimetric or enzymatic reaction, which is directly proportional to the amount of uric acid present in the sample.
The enzymatic method is preferred due to its specificity, sensitivity, and accuracy in measuring uric acid levels. It provides reliable results and is widely used in clinical laboratories for diagnosing and monitoring conditions such as gout, kidney stones, and certain metabolic disorders.
3: What are the 4 methods of uric acid determination?
Answer: There are several methods available for the determination of uric acid levels. Here are four commonly used methods:
- Enzymatic Method: This method involves the use of the enzyme uricase, which catalyzes the oxidation of uric acid to allantoin, carbon dioxide, and hydrogen peroxide. The generated hydrogen peroxide is then measured using a colorimetric or enzymatic reaction, and the intensity of the reaction is proportional to the uric acid concentration.
- Spectrophotometric Method: In this method, the absorbance or optical density of a specific wavelength of light is measured to determine the concentration of uric acid. It typically involves the use of a chromogenic reagent that reacts with uric acid to produce a colored product, the intensity of which is directly proportional to the uric acid concentration.
- High-Performance Liquid Chromatography (HPLC): HPLC is a technique commonly used for the separation and quantification of various compounds, including uric acid. In this method, a sample containing uric acid is injected into a high-pressure liquid chromatography system, where the compounds are separated based on their interactions with the stationary and mobile phases. Uric acid is then detected and quantified using a UV detector.
- Electrochemical Method: Electrochemical methods involve the use of electrodes to detect and quantify uric acid levels. The most common approach is amperometry, where a working electrode is employed to measure the current generated due to the oxidation or reduction of uric acid at a specific potential. The magnitude of the current is directly proportional to the uric acid concentration.
4: Which reagent is used for estimation of uric acid?
Answer: The reagent commonly used for the estimation of uric acid is the enzyme uricase. Uricase catalyzes the oxidation of uric acid to allantoin, carbon dioxide, and hydrogen peroxide. The enzymatic reaction can be coupled with colorimetric or enzymatic reactions to measure the generated hydrogen peroxide, which is directly proportional to the amount of uric acid present in the sample.
5: What is the pH of uric acid solution?
Answer: In its pure form, uric acid has a relatively low solubility in water, and its pH is typically around 5.5 to 6.0. However, when uric acid dissolves in water or bodily fluids, it can undergo partial dissociation, resulting in the release of hydrogen ions and a decrease in pH.
6: How do you reduce uric acid?
Answer: To reduce uric acid levels in the body, you can follow these general recommendations:
- Hydration: Drink plenty of water throughout the day to promote uric acid excretion through urine.
- Diet modifications: Limit or avoid high-purine foods such as organ meats, shellfish, red meat, and certain types of fish (e.g., anchovies, sardines) as they can increase uric acid production. Increase your intake of low-purine foods such as fruits, vegetables, whole grains, and low-fat dairy products.
- Limit alcohol consumption: Alcohol, especially beer, can increase uric acid levels and trigger gout attacks. Limit or avoid alcohol consumption, particularly beer and spirits.
- Weight management: Maintain a healthy weight or aim for gradual weight loss if overweight. Excess body weight can lead to increased uric acid production.
- Medications: In some cases, medications may be prescribed to help lower uric acid levels or manage gout symptoms. Consult a healthcare professional for appropriate medication options and guidance.
- Regular exercise: Engage in regular physical activity as it can help maintain overall health, control weight, and promote the excretion of uric acid.
7: What happens when your uric acid is high?
Answer: When your uric acid levels are high, it can lead to a condition called hyperuricemia. Hyperuricemia occurs when there is an excessive buildup of uric acid in the bloodstream. This can have several effects on the body, including:
- Gout: Hyperuricemia is a major risk factor for developing gout, a form of inflammatory arthritis. High levels of uric acid can cause urate crystals to form and accumulate in the joints, leading to sudden and severe joint pain, swelling, redness, and stiffness.
- Kidney stones: Uric acid can also form crystals in the kidneys, leading to the development of kidney stones. These stones can cause intense pain, blood in the urine, and urinary tract infections.
- Kidney damage: Prolonged high levels of uric acid can contribute to the development of kidney damage or kidney disease. Uric acid crystals can deposit in the kidneys and cause inflammation, impairing kidney function over time.
- Cardiovascular complications: Some studies suggest a link between high uric acid levels and an increased risk of cardiovascular diseases, such as hypertension, heart disease, and stroke. However, the exact mechanisms behind this association are still under investigation.
- Metabolic syndrome: Hyperuricemia is often associated with metabolic syndrome, a cluster of conditions that include obesity, high blood pressure, high blood sugar, and abnormal cholesterol levels. Together, these factors increase the risk of developing diabetes and heart disease.
8: What foods cause uric acid?
Answer: Certain foods are known to contribute to increased levels of uric acid in the body. These foods are typically high in purines, which are substances that break down into uric acid during digestion. Consuming excessive amounts of purine-rich foods can lead to elevated uric acid levels. Some common foods that can cause uric acid to increase include:
- Organ meats: Liver, kidney, and other organ meats are particularly high in purines.
- Seafood: Certain types of seafood, such as anchovies, sardines, mussels, scallops, and shrimp, contain high levels of purines.
- Red meat: Beef, lamb, and pork have moderate to high purine content, especially if they are processed or fatty cuts.
- Game meats: Wild game meats, such as venison, rabbit, and pheasant, have high purine levels.
- Yeast extracts: Foods like yeast extract spreads, yeast-based breads, and certain gravies or sauces made with yeast can be high in purines.
- Beer and alcoholic beverages: Beer, in particular, has been associated with increased uric acid levels due to the presence of purines and the way alcohol affects uric acid metabolism.




