Table of Contents
Aluminium chloride is a chemical compound with the formula AlCl3. It is an important compound with a wide range of applications in various industries. In this note, we will explore the formula and structure of aluminium chloride, as well as its chemical and physical properties.
Formula and Structure of Aluminium Chloride
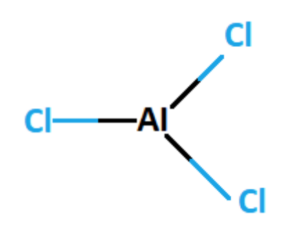
The formula AlCl3 indicates that aluminium chloride is composed of one aluminium (Al) atom and three chlorine (Cl) atoms. The aluminium atom loses three electrons, forming a positive charge (Al3+), while the chlorine atoms gain one electron each, forming negative charges (Cl–). The resulting ionic compound is held together by electrostatic attractions between the aluminium cation and chloride anions.
Aluminium Chloride Chemical Properties
- Lewis Acid: Aluminium chloride is known as a Lewis acid, meaning it can accept a pair of electrons to form a coordinate covalent bond. It readily reacts with Lewis bases, such as ethers and amines, to form adducts.
- Hydrolysis: Aluminium chloride undergoes hydrolysis when exposed to moisture or water. This reaction generates hydrochloric acid (HCl) and aluminium hydroxide (Al(OH)3). This property is significant in the industrial production of aluminium hydroxide, which is used in the manufacturing of various products, including ceramics and pharmaceuticals.
- Catalyst: Aluminium chloride acts as a catalyst in a variety of chemical reactions, including the Friedel-Crafts acylation and alkylation reactions. It facilitates the formation of carbon-carbon bonds and is commonly employed in the synthesis of organic compounds.
- Lewis Acid Chloride: Aluminium chloride can also function as a Lewis acid chloride, participating in substitution reactions. It reacts with organic compounds containing a nucleophilic group, such as alcohols and carboxylic acids, to form alkyl and acyl chlorides, respectively.
| Also Check | |
| Ammonia formula | |
| Ethanol formula | Oxalic acid formula |
Aluminium Chloride Physical Properties
- Appearance: Aluminium chloride exists as a white to pale yellow crystalline solid. It can also be found in a powdered form.
- Odour: Aluminium chloride has a strong, pungent odour, which can be unpleasant.
- Melting and Boiling Points: The melting point of aluminium chloride is approximately 180oC (356o Fahrenheit), while the boiling point is around 17 o C (352o Fahrenheit). It can sublime at elevated temperatures.
- Solubility: Aluminium chloride is highly soluble in polar solvents such as water and ethanol. The solubility in water increases with temperature. In nonpolar solvents, it has limited solubility.
- Conductivity: In its solid form, aluminium chloride does not conduct electricity. However, when dissolved in water or a molten state, it dissociates into ions, allowing it to conduct electricity.
Solved Examples related to the formula of sodium carbonate:
Example 1: Calculate the mass of sodium carbonate (Na2CO3) required to prepare 500 mL of a 0.1 M solution.
Solution: To calculate the mass of sodium carbonate needed, we need to use the formula:
Mass (g) = Molar concentration (mol/L) × Volume (L) × Molar mass (g/mol)
Given:
Molar concentration (M) = 0.1 M
Volume (L) = 0.5 L (since 500 mL is equal to 0.5 L)
Molar mass of Na2CO3 = 105.99 g/mol (calculated in a previous response)
Substituting the values into the formula:
Mass (g) = 0.1 mol/L × 0.5 L × 105.99 g/mol
= 5.3 g
Therefore, 5.3 grams of sodium carbonate is required to prepare 500 mL of a 0.1 M solution.
Example 2: How many moles of sodium carbonate are present in 250 grams of Na2CO3?
Solution: To determine the number of moles of sodium carbonate, we need to divide the given mass by the molar mass of Na2CO3.
Molar mass of Na2CO3 = 105.99 g/mol (calculated in a previous response)
Number of moles = Mass (g) / Molar mass (g/mol)
= 250 g / 105.99 g/mol
≈ 2.36 mol (rounded to two decimal places)
Therefore, there are approximately 2.36 moles of sodium carbonate in 250 grams of Na2CO3.
Frequently Asked Questions (FAQs)
What is the chemical formula of aluminium chloride?
The chemical formula of aluminium chloride is AlCl3. It indicates that each molecule of aluminium chloride contains one aluminium atom Al and three chlorine atoms Cl.
Is aluminium chloride an ionic or covalent compound?
Aluminium chloride is an ionic compound. It forms when the aluminium atom donates three electrons to chlorine atoms, resulting in the formation of Al3plus cations and Cl minus anions. The electrostatic attraction between these oppositely charged ions holds the compound together.
What is the molar mass of aluminium chloride?
The molar mass of aluminium chloride is calculated by adding the atomic masses of aluminium Al and chlorine Cl. The atomic mass of aluminium is approximately 26.98 g/mol, and the atomic mass of chlorine is about 35.45 g/mol. Thus, the molar mass of aluminium chloride is around 133.34 g/mol.
What is the structure of aluminium chloride?
Aluminium chloride exists as a solid crystalline compound, with each aluminium atom surrounded by six chloride atoms in an octahedral arrangement. The chloride ions are arranged around the central aluminium atom in a way that maximizes their electrostatic interaction.
What are the uses of aluminium chloride?
Aluminium chloride has various applications in different fields. It is commonly used as a catalyst in organic synthesis, especially in the Friedel-Crafts reaction. It also finds use in the production of dyes, pharmaceuticals, and fragrances. Additionally, aluminium chloride is employed in water treatment processes, as an ingredient in antiperspirants, and in the manufacture of aluminium metal.






