Table of Contents
Download PDF of important questions for Class 12 Physics Chapter 12 covers all key topics of the chapter. This chapter introduces students to modern physics concepts. The extra questions for Atoms and Nuclei in Class 12 offer a comprehensive understanding. Prepared by subject experts, these questions adhere to updated CBSE Syllabus guidelines. You can download more Important Questions Class 12 Physics Chapter 12 PDF for free from Infinity Learn -Learning App.
Download CBSE Class 12 Physics Important Questions 2023-24 PDF
Students can check CBSE Class 12 Physics Important Questions for other chapters:
| CBSE Class 12 Physics Important Questions | ||
| S.No | Chapter No | Chapter Name |
| 1 | Chapter 1 | Electric Charges and Fields |
| 2 | Chapter 2 | Electrostatic Potential and Capacitance |
| 3 | Chapter 3 | Current Electricity |
| 4 | Chapter 4 | Moving Charges and Magnetism |
| 5 | Chapter 5 | Magnetism And Matter |
| 6 | Chapter 6 | Electromagnetic Induction |
| 7 | Chapter 7 | Alternating Current |
| 8 | Chapter 8 | Electromagnetic Waves |
| 9 | Chapter 9 | Ray Optics and Optical Instruments |
| 10 | Chapter 10 | Wave Optics |
| 11 | Chapter 11 | Dual Nature of Radiation and Matter |
| 12 | Chapter 12 | Atoms |
| 13 | Chapter 13 | Nuclei |
| 14 | Chapter 14 | Semiconductor Electronic: Material, Devices And Simple Circuits |
| 15 | Chapter 15 | |
Important Questions for CBSE Class 12 Physics Atoms
1.All elements consists of very small invisible particles, called Every atom is a sphere of radius of the order of 10_10m, in which entire mass is uniformly distributed and negative charged electrons revolve around the nucleus.
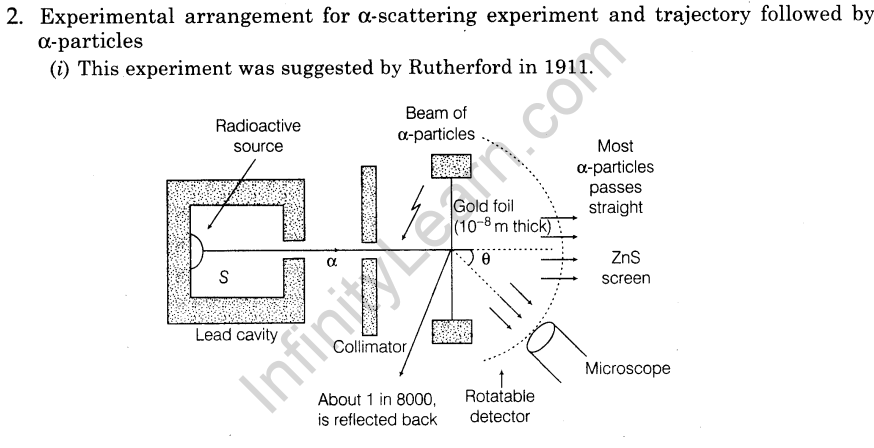
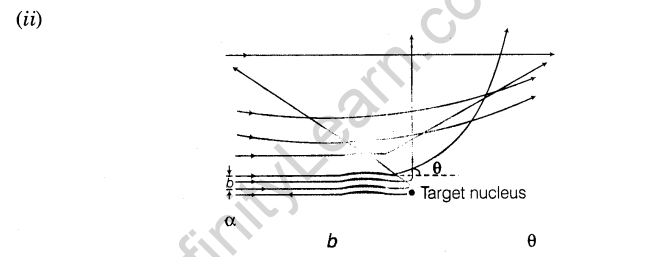
3.Impact parameter perpendicular distance of the velocity vector of a-particle from the central line of the nucleus of the atom is called impact parameter (b).
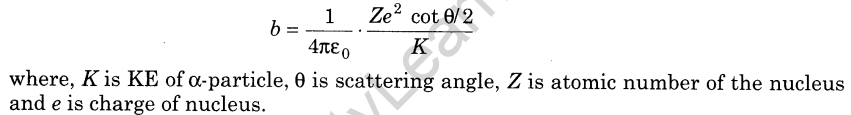
4.Basic assumption of Rutherford’s atomic model
- Atom consists of small central core, called atomic nucleus in which whole mass and positive charge is assumed to be concentrated.
- The size of nucleus is much smaller than the size of the atom.
- The nucleus is surrounded by electrons and atom is electrically neutral
Also Check: CBSE Class 12 Physics Syllabus 2024

6.Angle of Scattering Angle by which a-particle gets deviated from its original path around the nucleus is called angle of scattering.
7.Drawbacks of Rutherford’s Model
(i) Could not explained stability of atom clearly.
(ii) Unable to explain line spectrum
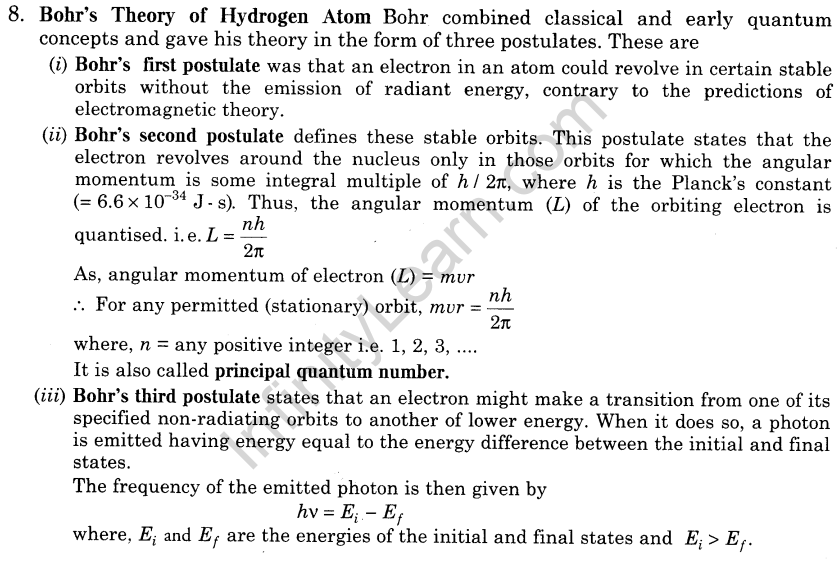
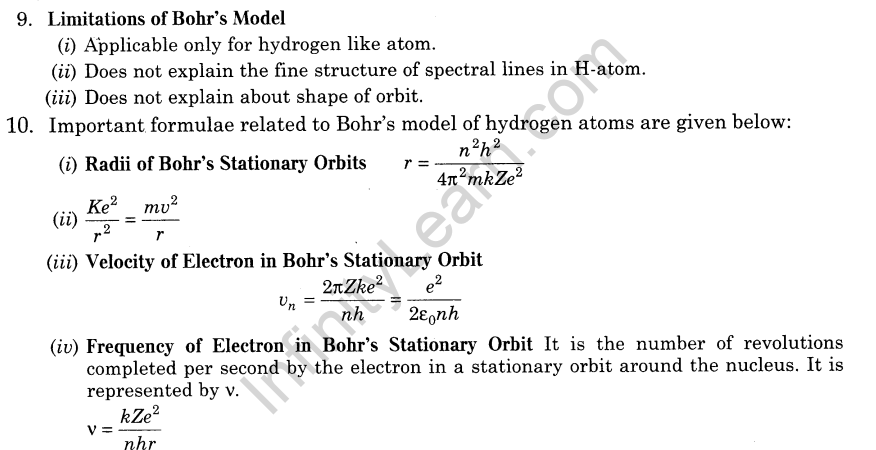
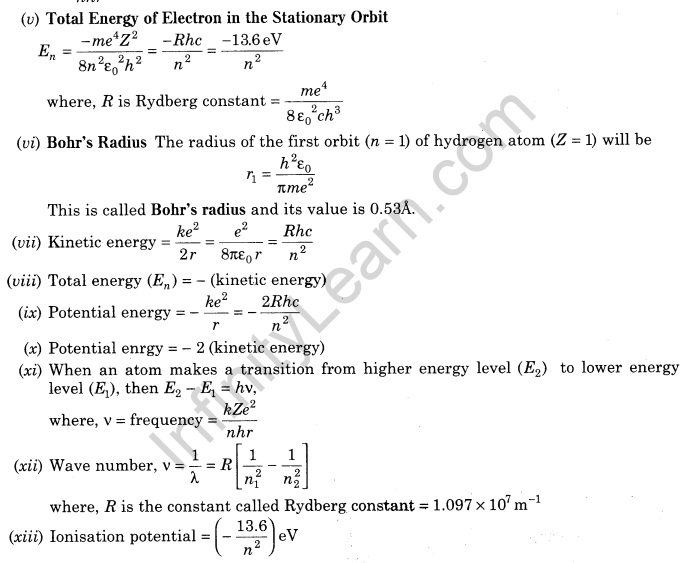
11.Energy Level The energy of an atom is the least when its electron is revolving in an orbit closest to the nucleus i.e. for which n = 1.
12.The lowest state of the atom is called the ground state, this state has lowest energy. The energy of this state is -13.6 eV. Therefore, the minimum energy required to free the electron from the ground state of the hydrogen atom is -13.6 eV.
13.(i)Emission Spectrum Hydrogen spectrum consists of discrete bright lines a dark background and it is specifically known as hydrogen emission spectrum.
(ii) Absorption Spectrum There is one more type of hydrogen spectrum exists where we get dark lines on the bright background, it is known as absorption spectrum
Read More: CBSE Class 12 Physics Answer Key 2024 | CBSE Class 12 Physics Paper Analysis 2024
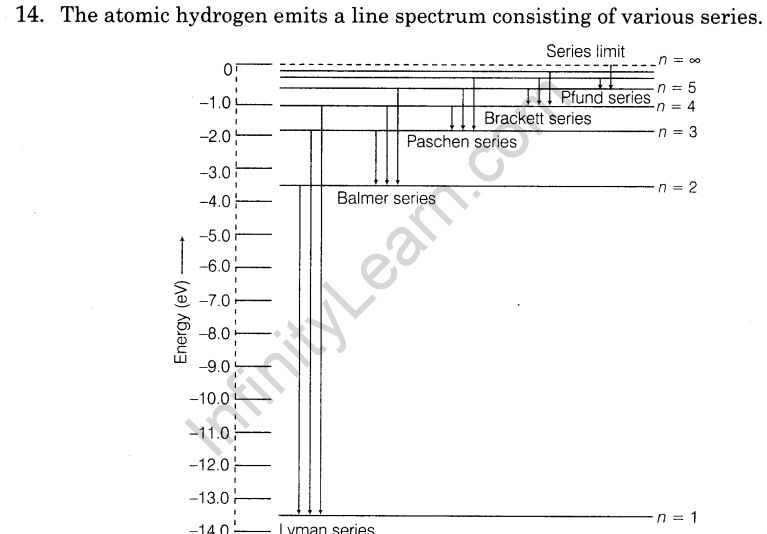
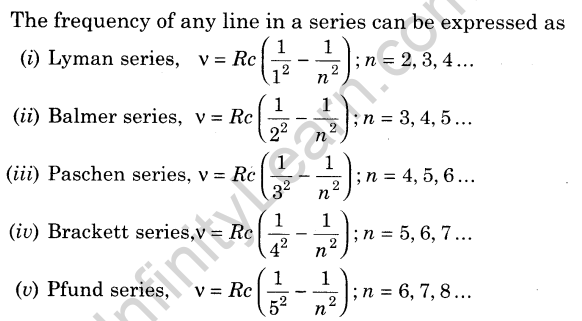
Previous Years Questions
1 Mark Questions
1.The ground state energy of hydrogen atom is – 13.6 eV. What are the kinetic and potential energies of electron in this state? [All India 2014C; HOTS; All India 2010]
Ans. Given, total ground state energy (TE) = (-13.6eV)
.-. Kinetic energy = – TE
= -(-13.6 eV) =13.6 eV Potential energy = 2 (TE)
= 2 x(-13.6) = -27.2 eV
2. When is Ha-line of the Balmer series in the emission spectrum of hydrogen atom obtained? [Delhi 2013C]
Ans. Ha-line of the Balmer series in the emission spectrum of hydrogen atom is obtained in visible region.
3. Why is the classical (Rutherford) model for an atom of electron orbitting around the nucleus not able to explain the atomic Structure? [Delhi 2012]
Ans. The classical method could not explain the atomic structure as the electron revolving around the nucleus are accelerated and emits energy as the result, the radius of the circular paths goes on decreasing. Ultimately electrons fall into the nucleus, which is not in practical.
4. Define ionisation energy. What is its value for a hydrogen atoms? [All India 2010]
Ans. Ionisation energy The minimum amount of energy required to remove an electron from the ground state of the atom is known as ionisation energy.
![]()
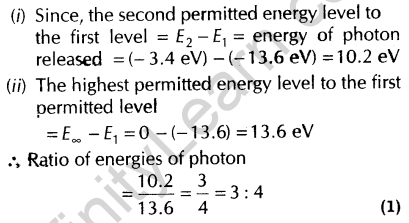
5. Find the ratio of energies of photons produced due to transition of an electron of hydrogen atom from its
- second permitted energy level to the first permitted level and
- the highest permitted energy level to the first permitted level. [All India 2010]
Ans.
6.What is the ratio of radii of the orbits corresponding to first excited state and ground state, in a hydrogen atom? [Delhi 2010]
Ans.
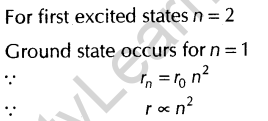

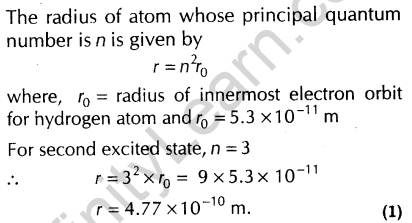
7. The radius of innermost electron orbit of a hydrogen atom is 5.3x 10-11 What is the radius of orbit in the second excited state?
Ans.
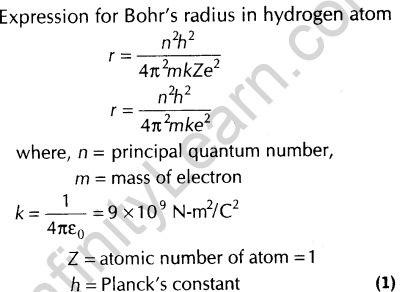
8.Write the expression for Bohr’s radius in hydrogen atom. [Delhi 2010]
Ans.
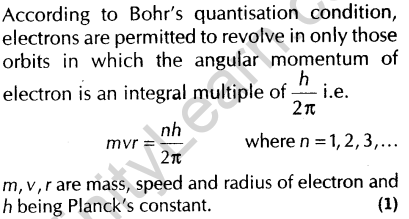
9. State Bohr’s quantisation condition for defining stationary orbits. [Foreign 2010]
Ans.
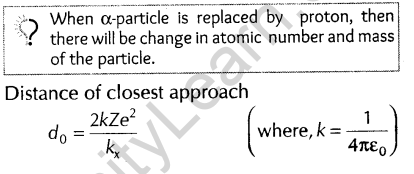
10. In the Rutherford scattering experiment, the distance of closest approach for an a-particle is do. If a-particle is replaced by a proton, then how much kinetic energy in comparison to a-particle will be required to have the same distance of Closest approach do ? [Foreign 2009]
Ans.