Table of Contents
Introduction to Zinc Nitrate Formula
Zinc nitrate is an inorganic compound with the chemical formula Zn(NO3)2. It is composed of:
- A zinc cation (Zn2+) and
- Two nitrate anions (NO3-).
The formula indicates that each zinc ion is associated with two nitrate ions.
Zinc nitrate is a white crystalline solid that is highly soluble in water. It is commonly used in various industries and applications, including as a mordant in textile dyeing, a corrosion inhibitor, a catalyst in chemical reactions, and a component in fertilizers. It can also be used in the synthesis of other zinc compounds.
The compound can be prepared by reacting zinc oxide or zinc metal with nitric acid. The reaction results in the formation of zinc nitrate and water:
ZnO + 2HNO3 → Zn(NO3)2 + H2O
Structural Formula of Zinc Nitrate
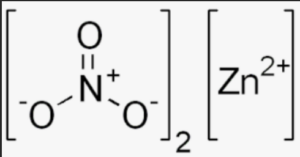
The structural formula of zinc nitrate is [Zn(NO3)2]. It represents the arrangement of atoms and their bonds in the molecule. In the case of zinc nitrate, the central zinc ion (Zn) is surrounded by two nitrate ions (NO3), forming a coordination compound. Each nitrate ion is composed of one nitrogen atom (N) bonded to three oxygen atoms (O) through single bonds and one of the oxygen atoms forming a double bond with the nitrogen atom.
Uses of Zinc Nitrate
- Laboratory Reagent: Zinc nitrate is used as a reagent in various laboratory experiments and chemical reactions. It is often utilized for the synthesis of other zinc compounds or as a source of zinc ions in solution.
- Galvanizing: Zinc nitrate is used in the galvanizing process, where it acts as a precursor for zinc coatings on steel and other metal surfaces. The zinc coating provides protection against corrosion, increasing the lifespan and durability of the metal.
- Agriculture: Zinc nitrate is sometimes used as a fertilizer in agriculture to supply plants with essential zinc nutrients. Zinc is necessary for various plant functions, including growth, development, and enzyme activity. Zinc-deficient soils or crops can benefit from zinc nitrate application.
- Textile Industry: Zinc nitrate is employed in the textile industry as a mordant in dyeing and printing processes. It helps improve the colorfastness and bonding of dyes to the fabric, resulting in vibrant and long-lasting colors.
- Water Treatment: Zinc nitrate can be used in water treatment applications, specifically for controlling algae and inhibiting the growth of certain microorganisms. It helps maintain water quality and prevents biological fouling in systems such as cooling towers and swimming pools.
- Catalysts and Chemical Reactions: Zinc nitrate can serve as a catalyst or catalyst precursor in various chemical reactions. It is used in organic synthesis, oxidation reactions, and in the production of other chemicals.
Physical Properties of Zinc Nitrate Formula
- Appearance: Zinc nitrate can be a white crystalline solid or a colorless solution, depending on its form and concentration.
Solubility: It is highly soluble in water, meaning it readily dissolves in water to form a solution. - Hydration: Zinc nitrate can exist in different hydration states, meaning it can incorporate water molecules into its structure. The most common form is the hexahydrate (Zn(NO3)2·6H2O), which contains six water molecules per formula unit.
- Melting and Boiling Points: The melting point of zinc nitrate hexahydrate is approximately 36 degrees Celsius (97 degrees Fahrenheit), while the anhydrous form of zinc nitrate (Zn(NO3)2) has a higher melting point.
- Density: The density of zinc nitrate can vary depending on its form, concentration, and temperature. The density typically ranges from about 2.065 to 2.065 grams per cubic centimeter.
Chemical Properties of Zinc Nitrate Formula
- Solubility: Zinc nitrate is highly soluble in water. When it is dissolved in water, it dissociates into zinc ions (Zn2+) and nitrate ions (NO3-). This solubility makes it useful in various applications, such as in the preparation of zinc-containing solutions or as a precursor for other zinc compounds.
- Acidic Nature: Zinc nitrate is a source of nitrate ions (NO3-), which can act as an acid when dissolved in water. It can undergo protonation reactions, releasing hydrogen ions (H+) into solution. Thus, zinc nitrate solutions have acidic properties.
- Oxidizing Agent: In certain reactions, zinc nitrate can act as an oxidizing agent. It can donate oxygen atoms or accept electrons, facilitating the oxidation of other substances. This property is particularly relevant in redox reactions and can be utilized in various chemical processes.
- Decomposition: When heated to high temperatures, zinc nitrate decomposes to produce nitrogen dioxide gas (NO2), oxygen gas (O2), and zinc oxide (ZnO). The decomposition reaction is as follows: 2Zn(NO3)2 → 2ZnO + 4NO2 + O2
- Reaction with Alkalis: Zinc nitrate can react with alkalis, such as sodium hydroxide (NaOH), to form zinc hydroxide (Zn(OH)2) and sodium nitrate (NaNO3). The balanced chemical equation for this reaction is: Zn(NO3)2 + 2NaOH → Zn(OH)2 + 2NaNO3
Conclusion
In conclusion, zinc nitrate (Zn(NO3)2) is a compound that has several practical uses. It is commonly employed as a precursor in the synthesis of other zinc compounds, including zinc oxide, zinc hydroxide, and zinc carbonate.
Zinc nitrate is also utilized in the manufacturing of catalysts, dyes, and pigments. In addition, it finds application in the field of agriculture as a source of zinc nutrient for plants. Zinc is an essential micronutrient for plant growth and development, and zinc nitrate can be used as a foliar spray or soil amendment to address zinc deficiency in crops.
Furthermore, zinc nitrate has applications in the production of fireworks and pyrotechnics, where it acts as a component in the formulation of colored flames. Its ability to provide a green color to flames makes it a valuable ingredient in such applications. Overall, zinc nitrate has diverse uses in various industries, ranging from chemical synthesis to agriculture and pyrotechnics
Solved Examples for Zinc Nitrate
Example 1: Calculate the molar mass of zinc nitrate.
Solution: The molar mass of zinc (Zn) is 65.38 g/mol.
The molar mass of nitrate (NO3) is calculated as follows:
Nitrogen (N) has a molar mass of 14.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Since there are three oxygen atoms in nitrate, the total molar mass of nitrate is
14.01 + (3 * 16.00) = 62.01 g/mol.
The molar mass of zinc nitrate (Zn(NO3)2) is: (1 * 65.38) + (2 * 62.01)
= 65.38 + 124.02
= 189.40 g/mol.
Example 2: How many moles of zinc nitrate are present in 250 grams of the compound?
Solution: We need to convert the mass of zinc nitrate to moles using its molar mass.
Molar mass of zinc nitrate (Zn(NO3)2) = 189.40 g/mol.
Number of moles = Mass (g) / Molar mass (g/mol)
Number of moles = 250 g / 189.40 g/mol
Number of moles = 1.320 moles (rounded to three decimal places).
More Formulas:
| Urea formula | |
| Ethanol formula | Oxalic acid formula |
| Nitric acid formula | Benzene formula |
| Ammonia formula | Acetone formula |
| Methane formula | Calcium carbonate formula |
Frequently Asked Questions on Zinc nitrate
What is the formula for zinc nitrate?
The chemical formula of zinc nitrate is Zn(NO3)2.
What is the importance of zinc nitrate?
Zinc Nitrate is a colorless or white, odorless, crystalline (sand- like) solid or flake. It is used as a catalyst and a mordant for dyes, and in liquid fertilizer. It can also be used in the synthesis of other zinc compounds.
Is zinc nitrate acidic or basic?
Zinc nitrate is a salt of a strong acid and a weak base. On ionisation, it yields nitrate and zinc ions in water. Hydrolysis of the basic radical, zinc ion, gives rise to the weak base zinc hydroxide and hydrogen ions, making the solution acidic.
What happens when zinc nitrate reacts with NaOH?
Sodium hydroxide (NaOH) is added to zinc nitrate (Zn(NO3)2). The result is a white precipitate.
Does zinc nitrate dissolve in water?
Zinc Nitrate is a highly water soluble crystalline Zinc source for uses compatible with nitrates and lower (acidic) pH. Nitrate compounds are generally soluble in water.
What are the hazards of zinc nitrate?
Exposure to Zinc Nitrate can cause headache, dizziness, nausea and vomiting. Exposure to very high levels may cause trouble breathing, collapse and even death.








