Table of Contents
Introduction to Aluminium Oxide
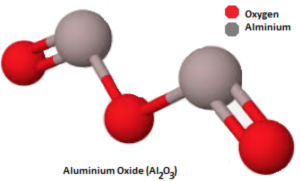
Aluminium oxide, also known as alumina, is a chemical compound with the formula Al₂O₃.
Formula and Structure of Aluminium Oxide
The chemical formula of aluminium oxide is Al₂O₃. It consists of two aluminium atoms (Al₂) and three oxygen atoms (O₃), arranged in a lattice structure.
Physical Properties of Aluminium Oxide
- Molecular Weight: The molecular weight of aluminium oxide is approximately 101.96 g/mol.
- State: Aluminium oxide exists as a solid at room temperature.
- Appearance: It is a white or off-white powder or granular material.
- Melting Point: Aluminium oxide has a high melting point of around 2,072°C (3,762°F).
- Density: The density of aluminium oxide ranges from 3.95 to 4.1 g/cm³, depending on its crystal structure.
- Insolubility: Aluminium oxide is insoluble in water and most organic solvents.
Chemical Properties of Aluminium Oxide
- Refractory Nature: Aluminium oxide is a refractory material, meaning it has a high resistance to heat and can withstand extremely high temperatures.
- Corrosion Resistance: Aluminium oxide is highly corrosion-resistant, making it useful for applications in which exposure to harsh environments or chemicals is expected.
- Insulating Properties: Aluminium oxide is an electrical insulator, meaning it does not conduct electricity.
- Acid-Base Reactivity: Aluminium oxide can react with both acids and bases. It reacts with acids to form salts and water. However, it is less reactive compared to other metal oxides. Example: Al₂O₃ + 6HCl → 2AlCl₃ + 3H₂O
- Catalyst Support: Aluminium oxide is commonly used as a catalyst support material, providing a stable structure for catalysts in various chemical reactions.
- Abrasive Properties: Aluminium oxide is widely used as an abrasive material in applications such as sandpaper, grinding wheels, and polishing compounds due to its hardness and durability.
Aluminium oxide has numerous industrial applications, including in the production of metals, ceramics, refractories, abrasives, electrical insulators, and catalysts.
Frequently Asked Questions of Aluminium Oxide
What is aluminium oxide used for?
Aluminium oxide is used in many things, like making ceramics, polishing materials, and even as an ingredient in some medicines.
What is Al2O3 called?
Al2O3 is called aluminium oxide, and it's a compound made of aluminum and oxygen.
Why is the formula Al2O3?
The formula Al2O3 shows that there are two atoms of aluminum and three atoms of oxygen in each molecule of aluminium oxide.
What is aluminium oxide called?
Aluminium oxide is also known as alumina.
What are the physical properties of alumina?
Alumina is hard, has a high melting point, and is a good electrical insulator.
Is aluminium oxide a strong acid?
No, aluminium oxide is not a strong acid. It's actually a type of compound called an oxide
What are the 3 physical properties of an aluminum can?
An aluminum can is lightweight, durable, and doesn't rust easily.






