Table of Contents
Calcium Oxide Formula
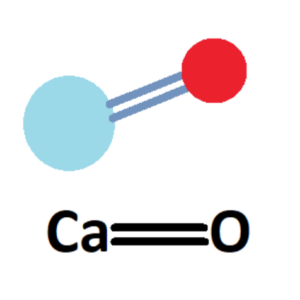
Calcium oxide, also known as quicklime or burnt lime, has the chemical formula CaO. It consists of one calcium cation (Ca2+) and one oxygen anion (O2-), which are held together by ionic bonds.
The molecular structure of calcium oxide can be represented as a simple ionic lattice. In this lattice structure, each calcium cation is surrounded by six oxygen anions, and each oxygen anion is surrounded by six calcium cations. This arrangement forms a crystal lattice.
Calcium Oxide Formula: Physical Properties
- Appearance: Calcium oxide is a white, crystalline solid with a powdery texture. It is often found in the form of granules or powder.
- Melting Point: It has a high melting point of approximately 2,572 degrees Celsius (4,662 degrees Fahrenheit).
- Density: The density of calcium oxide is around 3.34 grams per cubic centimeter.
- Solubility: Calcium oxide is sparingly soluble in water. When it reacts with water, it undergoes a chemical reaction to form calcium hydroxide, also known as slaked lime.
Calcium Oxide Formula: Chemical properties
- Reactivity with Water: Calcium oxide is highly reactive with water, undergoing a vigorous exothermic reaction known as hydration. During this reaction, calcium oxide combines with water to form calcium hydroxide. This reaction is often used in applications where the generation of heat is desired, such as in self-heating meals or hand warmers.
- Acid-Base Properties: Calcium oxide is a strong base and can neutralize acids. It reacts with acids to form calcium salts and water. This property makes it useful in various industrial processes, such as wastewater treatment and pH adjustment.
- Dehydrating Agent: Calcium oxide has a strong affinity for water and can act as a desiccant or dehydrating agent. It readily absorbs moisture from the air, making it useful in applications where drying or removing moisture is essential, such as in the production of drying agents or as a component in desiccant packs.
- Construction Material: Calcium oxide is an important component in the production of cement and mortar. It reacts with water to form calcium hydroxide, which then reacts with atmospheric carbon dioxide to form calcium carbonate. This process, known as carbonation, contributes to the hardening and strength of concrete structures.
- Desulfurization: Calcium oxide is used in the desulfurization of industrial flue gases. It reacts with sulfur dioxide (SO2) to form calcium sulfite, which can then be oxidized to calcium sulfate. This process helps remove harmful sulfur emissions and reduce environmental pollution.
Solved Examples on Calcium oxide Formula
Example 1. What is the molar mass of calcium oxide (CaO)?
Solution:
To calculate the molar mass of calcium oxide, we need to determine the atomic masses of calcium (Ca) and oxygen (O) from the periodic table. The atomic mass of calcium is approximately 40.08 g/mol, and the atomic mass of oxygen is approximately 16.00 g/mol. Since there is one calcium atom and one oxygen atom in calcium oxide, we can add their atomic masses together:
Molar mass of CaO = (atomic mass of Ca) + (atomic mass of O)
= 40.08 g/mol + 16.00 g/mol
= 56.08 g/mol
Therefore, the molar mass of calcium oxide is approximately 56.08 g/mol.
Example 2. What is the common name for calcium oxide?
Answer: Calcium oxide is commonly known as quicklime or burnt lime. These names refer to the traditional method of producing calcium oxide by heating calcium carbonate (such as limestone) to a high temperature, resulting in the decomposition of the carbonate and the formation of calcium oxide.
Frequently Asked Questions on Calcium Oxide Formula
What is the chemical formula for calcium oxide?
The chemical formula for calcium oxide is denoted as CaO, representing the ratio of calcium and oxygen atoms present in the compound.
What is the common name for calcium oxide?
The common name for calcium oxide is Quicklime. It's a term that has been used historically due to its swift reactivity with water.
What is the formula for CaO?
The formula CaO represents calcium oxide, showcasing the elemental composition of one calcium atom bonded to one oxygen atom.
Why CaO is called quicklime?
CaO is termed as quicklime due to its immediate reactive nature with water, forming slaked lime which is also known as calcium hydroxide.
What is the calcium oxide?
Calcium oxide is a white, caustic, alkaline crystalline solid at room temperature. It's well known for its extensive use in various industries.
What is the state of matter of calcium oxide at room temperature?
Calcium oxide is found in a solid state at room temperature. It's a white, caustic, and alkaline crystalline material.
What is the main use of calcium oxide?
The primary use of calcium oxide is in manufacturing cement, steel, and in water treatment, making it a crucial industrial chemical.
What is the Colour of CaO?
Calcium oxide, denoted as CaO, is white in color. Its white coloration is consistent and helps in its identification in industrial uses.
Is calcium oxide soluble in water?
Yes, calcium oxide is soluble in water. When mixed with water, it reacts vigorously to form calcium hydroxide, releasing a significant amount of heat.






