Table of Contents
Carbonic Acid Formula
Carbonic acid is a weak acid with the chemical formula H2CO3. In this note, we will explore the formula and structure of carbonic acid, as well as its chemical and physical properties.
Formula and Structure of Carbonic Acid
The formula for carbonic acid is H2CO3. It indicates that each molecule of carbonic acid contains two hydrogen (H) atoms, one carbon (C) atom, and three oxygen (O) atoms. The structure of carbonic acid is best represented as a central carbon atom bonded to two hydroxyl (OH) groups and a double-bonded oxygen atom.
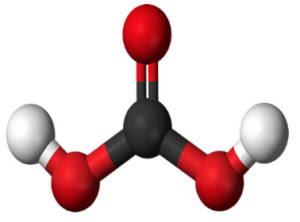
(carbonic acid) H2CO3
Chemical Properties of Carbonic Acid
- Acidic Nature: Carbonic acid is a weak acid that can undergo partial dissociation in water, releasing hydrogen ions (H+) and bicarbonate ions (HCO3-). The equilibrium between carbonic acid and its dissociated forms plays a vital role in maintaining the pH balance in various biological systems.
- Role in Carbon Dioxide Transport: Carbonic acid is an intermediate compound in the transportation of carbon dioxide (CO2) in the bloodstream. It forms when CO2 reacts with water (H2O) in red blood cells and is then transported to the lungs for removal.
- Decomposition: Carbonic acid is unstable and can decompose into water and carbon dioxide. This decomposition process is often accelerated by heat or the presence of catalysts.
Physical Properties of Carbonic Acid
- Appearance: Pure carbonic acid is a colorless liquid. However, it is usually encountered in its aqueous form, which is also colorless.
- Solubility: Carbonic acid is highly soluble in water, as it readily reacts with water molecules to form H+ and HCO3- ions. The solubility of carbonic acid increases with lower temperatures and higher pressures.
- Density: The density of carbonic acid is approximately 1.67 g/cm³. However, it is important to note that carbonic acid is most commonly encountered in dilute solutions rather than its pure form.
- Boiling Point: Carbonic acid does not have a well-defined boiling point since it decomposes before reaching a boiling temperature. The decomposition occurs around 100°C (212°F), resulting in the release of carbon dioxide and water.
- Stability: Carbonic acid is relatively unstable and tends to decompose into carbon dioxide and water. This decomposition reaction is reversible and depends on factors such as temperature, pressure, and the presence of catalysts.
Solved Examples on Carbonic acid Formula (H2CO3)
Example 1: Calculate the total number of atoms in one molecule of carbonic acid.
Solution:
The formula for carbonic acid is H2CO3. To calculate the total number of atoms in one molecule, we need to sum up the number of atoms for each element present in the formula.
Number of hydrogen atoms (H) = 2
Number of carbon atoms (C) = 1
Number of oxygen atoms (O) = 3
Total number of atoms in one molecule of carbonic acid = Number of hydrogen atoms + Number of carbon atoms + Number of oxygen atoms
= 2 + 1 + 3
= 6
Therefore, there are 6 atoms in one molecule of carbonic acid.
Example 2: Write the balanced chemical equation for the decomposition of carbonic acid into water and carbon dioxide.
Solution:
The decomposition reaction of carbonic acid can be represented by the following balanced chemical equation:
H2CO3 (aq) → H2O (l) + CO2 (g)
This equation indicates that one molecule of carbonic acid decomposes into one molecule of water and one molecule of carbon dioxide. The (aq) notation denotes that carbonic acid is dissolved in water, (l) denotes liquid water, and (g) denotes gaseous carbon dioxide.
Please note that the decomposition of carbonic acid is a reversible reaction, and the equation shown represents the decomposition in one direction.
Frequently asked Questions on Carbonic acid Formula (H2CO3)
How do I write a salary increment letter?
To write a salary increment letter, use a formal format. Start with a polite introduction, state your achievements and contributions, provide reasons for the raise, and suggest a new salary. Thank your employer and express your eagerness to discuss further.
How do you say increment in salary?
You can say increment in salary using terms like salary raise, pay increase, or wage hike. These phrases convey the idea of a salary increase clearly.
How do I request salary increment for staff?
When requesting a salary increment for staff, gather performance data, highlight their achievements, and present a strong case. Use a formal letter format, like the salary increment request letter, and be clear about the amount you're requesting.
How do I approach my boss for salary increment?
Approach your boss for a salary increment by scheduling a private meeting. Prepare a compelling case that includes your achievements, contributions, and industry standards. Politely and confidently express your request during the meeting.
How much raise should I ask for?
The raise you ask for should be based on factors like your performance, responsibilities, and market standards. A common range is 5-20%, but research industry averages and consider your specific situation to determine the right amount.
Why should we increase your salary?
Your salary should be increased based on your achievements, contributions, and the value you bring to the company. Demonstrating your impact on the organization and industry benchmarks can justify a raise.
Is it okay to ask for a 30% raise?
Asking for a 30% raise can be acceptable in exceptional cases where your performance and contributions warrant such a significant increase. However, it's crucial to justify this request with strong evidence.
How do you negotiate a pay rise?
Negotiating a pay rise involves research, preparation, and confident communication. Present your achievements and industry data, express your request politely, and be open to compromise.
Why you should write a Salary Increment Letter
Writing a Salary Increment Letter is essential to formalize your request or acknowledgment of a salary increase. It helps provide a clear and documented record of the salary adjustment, ensuring both parties are on the same page.








