Table of Contents
Learning the fundamental differences between acid and base is essential for students diving into the world of chemistry. By understanding what is acid and base, you can grasp how these substances interact with our everyday lives.
A key concept is exploring what do all acids and all bases have in common and how they uniquely react with each other. This knowledge not only enhances your grasp of chemistry but also lays the foundation for more advanced scientific concepts. So, let’s delve into the fascinating world of acids and bases, their reactions, and their distinctive characteristics.
What is Acid?
An acid is a substance that can donate a hydrogen ion (H+) to another substance. Acids are known for their sour taste and their ability to turn blue litmus paper red. They react with bases to form salt and water, showcasing how do acids and bases react with each other. This reaction is fundamental in understanding what is acid and base.
Advanced definition of Acid: Acid, as defined by the Arrhenius theory, is a substance that releases hydrogen ions (H+) when dissolved in water. This definition is primarily useful for aqueous solutions. When acids react with bases, they neutralize each other, as hydrogen ions combine with hydroxide ions to form water, represented by the reaction H+ (aq) + OH- (aq) → H2O (l).
Expanding on Arrhenius’ concept, the Bronsted theory identifies acids as proton donors, meaning they donate hydrogen ions. This definition includes not just typical acids but also substances like amines and alcohols. The Brønsted-Lowry acid concept is widely accepted as the most comprehensive definition of acids.
The Lewis theory takes a different approach, defining acids as electron pair acceptors. In this perspective, an acid is a substance that completes its outer shell by accepting electron pairs into its valence shell during a reaction, without necessarily changing its oxidation state.
Properties of Acids
- They conduct electricity, hence are electrolytes.
- They turn blue litmus paper red.
- Many acids are solid.
- Acids typically have a sour taste.
- They produce hydrogen gas when reacting with active metals like zinc, magnesium, aluminum, or iron.
- Acids have a low pH value, below 7.
- When reacting with bases or alkalis, they produce salts and water.
- Some acids are corrosive.
- Weak and more volatile acids can be displaced by stronger, less volatile acids in salts.
Acids can be categorized into two types based on their ion dissociation in water:
- Strong Acids: These completely dissociate into ions in water. Examples include hydrochloric acid (HCl), nitric acid (HNO3), hydrobromic acid (HBr), hydroiodic acid (HI), perchloric acid (HClO4), and chloric acid (HClO3).
- Weak Acids: These only partially dissociate into ions in water. Most acids fall into this category. An example is hydrofluoric acid, which is a weak acid despite being very strong and corrosive.
What is Base?
A base is a substance that can accept a hydrogen ion (H+) from an acid. Bases have a bitter taste and a slippery feel, and they turn red litmus paper blue. This is a key aspect in understanding what is acid and base and what do all acids and all bases have in common.
Advanced definition of Base: A base is a chemical entity that can donate electrons, accept protons, or release hydroxide ions (OH-) in water. Bases have certain characteristics that make them identifiable, such as a slippery feel (like soap), a bitter taste, and their ability to react with acids to form salts and catalyze certain reactions.
Different scientific models describe bases: Arrhenius bases, Bronsted-Lowry bases, and Lewis bases, each providing a unique perspective on what constitutes a base. Examples of bases include metal hydroxides like those of alkaline and alkaline earth metals, as well as substances like soap.
Properties of Bases
- They have a pH greater than 7.
- Bases taste bitter, although tasting them is not recommended for safety reasons.
- Bases can conduct electricity when dissolved or molten, as they dissociate into ions.
- Strong bases are caustic, reacting vigorously with acids and organic materials.
- They affect pH indicators in specific ways: turning litmus paper blue, methyl orange to yellow, phenolphthalein pink, while Bromothymol blue remains blue in their presence.
Bases are categorized into different types:
- Strong Bases: These completely dissociate into ions in water. They are also capable of removing a proton (H+) from a weak acid. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
- Weak Bases: These do not completely dissociate in water. Their aqueous solutions contain the base and its conjugate acid.
- Neutral Bases: These form bonds with neutral acids.
- Super Bases: Formed from an alkali metal and its conjugate acid, super bases are stronger than strong bases. An example is sodium hydride (NaH).
- Solid Bases: Active in solid form, these can be used in anion exchange resins or react with gaseous acids. Examples include silicon dioxide (SiO2) and NaOH on alumina.
Differences Between Acid and Base
In Class 10, understanding the difference between acid and base is crucial. Acids and bases differ in taste, reaction with litmus, texture, their reaction with metals, and pH values. This knowledge helps in comprehending what is the difference between an acid and a base, and how do acids and bases react with each other. Refer to the table below for the difference between acid and base –
| Basis | Acid | Base |
| Definition | A chemical compound that, when dissolved in water, produces a solution with more hydrogen ions than pure water. | An aqueous substance that can absorb hydrogen ions. |
| Strength | Depends on the concentration of hydronium ions (H3O+). | Depends on the concentration of hydroxide ions (OH-). |
| Examples | Acetic acid (CH3COOH), Sulphuric acid (H2SO4). | Sodium Hydroxide (NaOH), Ammonia (NH3). |
| Physical Characteristics | Can be solid, liquid, or gaseous, with a sour taste. | Typically solid and slippery (except for ammonia, which is gaseous), with a bitter taste. |
| Disassociation in Water | Releases hydrogen ions (H+) in water. | Releases hydroxide ions (OH-) in water. |
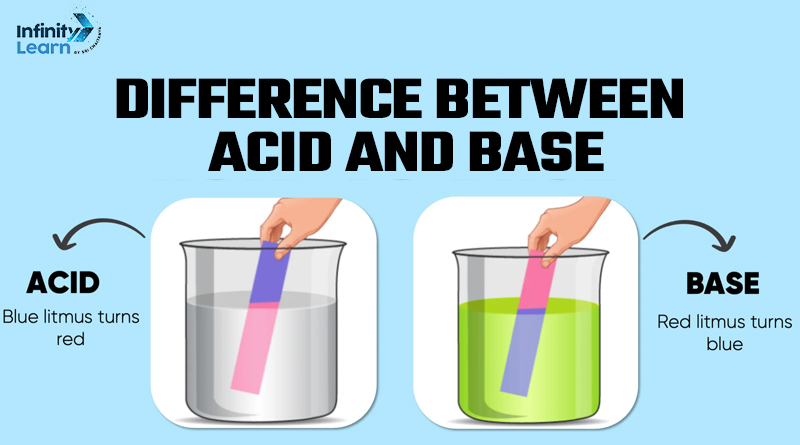
| Litmus Test Reaction | Turns blue litmus paper red. | Turns red litmus paper blue. |
The difference between acid and base extends beyond the classroom, playing a significant role in various scientific fields. Remember, the difference between acid and base class 10 is not just a curriculum topic but a stepping stone to deeper scientific understanding.
FAQs on Difference Between Acid and Base
What are the 5 difference between acid and base?
Taste: Acids are sour, while bases are bitter. pH Value: Acids have a pH lower than 7, while bases have a pH higher than 7. Color Change: Acids turn blue litmus paper red, while bases turn red litmus paper blue. Ion Donation: Acids donate H+ ions, while bases accept H+ ions. Reactivity: Acids react with metals to produce hydrogen gas, while bases react with some metals (like Al and Zn) to produce hydrogen gas.
What defines an acid vs a base?
Acids: Donate H+ ions (Brønsted-Lowry definition) or accept electron pairs (Lewis definition). Bases: Accept H+ ions (Brønsted-Lowry definition) or donate electron pairs (Lewis definition).
Is NaOH a base or an acid?
NaOH is a base. It readily accepts H+ ions and has a pH greater than 7. It can turn red litmus paper blue and neutralize acids.








