Table of Contents
One of the most important questions in exams is what is the difference between an element and a compound. In this article, we will discuss the difference between elements and compounds based on different parameters. Before studying the difference between elements and compounds it is necessary to know what are elements and compounds.
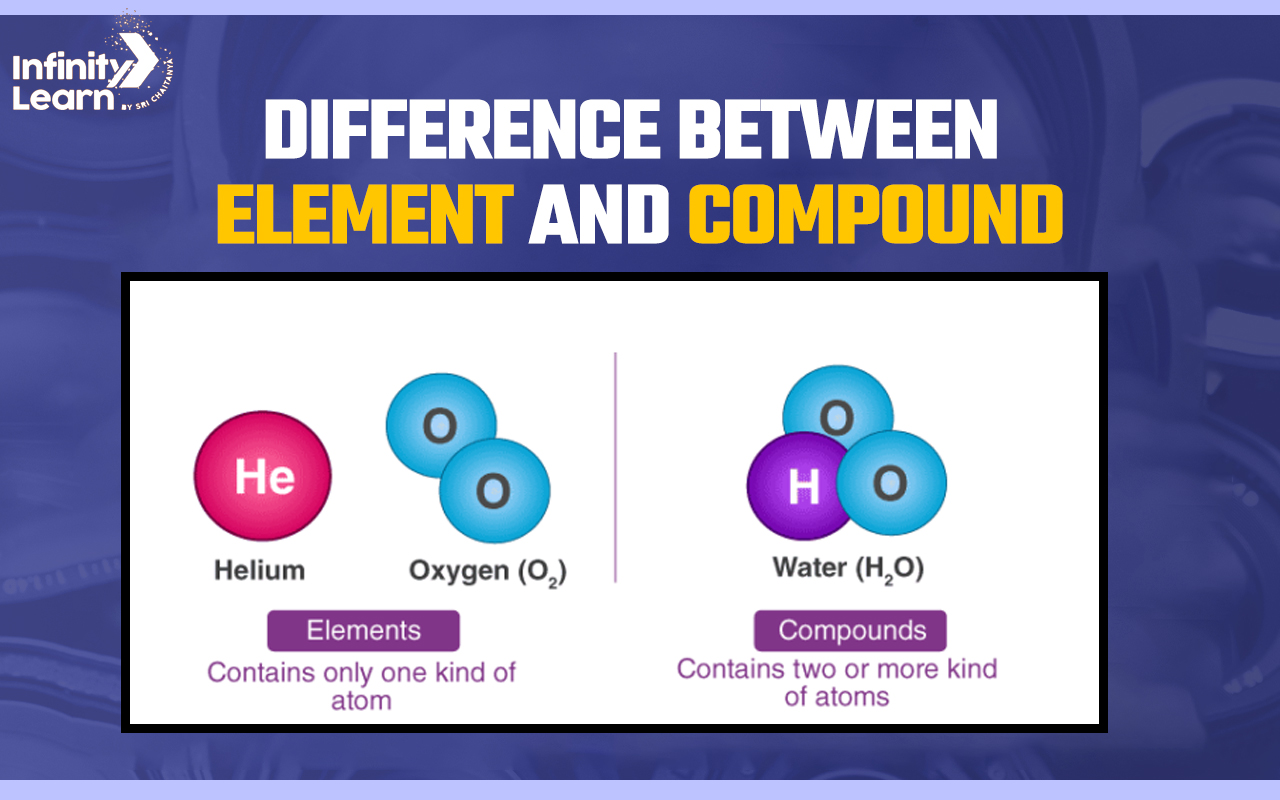
What are Elements and Compounds
Both elements and compounds are similar concepts. Elements are composed of only one type of substance whereas compounds are composed of two or more than two types of substance. Below given is the detailed difference between these both.
What is an Element?
Those materials which contain only one type of atom are called an element. These atoms have the same atomic number. For example – hydrogen, oxygen, and nitrogen.
There are three different types of chemical elements. These are metals, nonmetals and metalloids.
- Metals: Metals can be found on the left side of the periodic table. They have properties like electroconductivity, malleability, shine, etc. For example – copper, aluminium, gold etc.
- Non-metals: Non-metals are placed on the right side of the periodic table. They don’t have properties of malleability, electric conductivity and shiny. For example, oxygen and carbon.
- Metalloids: These are present in the middle of the periodic table. They have mixed properties of metals and non-metals. For example, germanium, silicon, etc.
What is a Compound?
Those substances formed by combining two or more elements are called compounds. These two elements are different from each other.
Elements in a compound are held together with covalent or ionic bonds. There are different types of compounds, as written below:
- Molecular compounds: In molecular compounds, molecules are held together by covalent bonds. For example: O2.
- Intermetallic compounds: Metallic bonds are formed between the molecules in these compounds. For example: Sendust
- Ionic compounds: The atoms bind each other with ionic bonds. For example, NaCl.
- Coordinate compounds: Molecules in coordinate compounds are held together by coordinate covalent bonds. For example, hemoglobin and chlorophyll.
Difference between Elements and Compounds
| Difference Between Elements And Compounds | ||
| Parameters | Elements | Compounds |
| Definition | Elements comprise a single type of atom. | Compounds are products of two or more than two types of atoms. |
| Total numbers | There are 118 elements found on the earth. | Number of compounds is infinite. There is no fixed number. |
| Classification | Metals, nonmetals and metalloids. | Molecular, intermetallic, ionic and coordinate. |
| Representation | Chemical symbols are used to represent elements. | Chemical formula is used to represent a compound. |
| Ability to breakdown | Elements cannot be broken down with any chemical reaction. | Compounds can be broken down and can be converted into new forms or compounds with the help of chemical reaction. |
| Structure | Elements are pure substances. | Elements are chemically combined. |
| Bonding | There is absence of covalent or ionic bonds between the atoms | Compounds are formed with the covalent or ionic bonds present between the atoms |
| Charge | Atoms are of neutral charge. | Ions are formed. |
| Availability | These are available in nature. | Some compounds are freely available while most of them are man made. |
| Representation | Elements are represented with symbols. | Compounds are represented using a formula. |
| Separation | Separation of elements can be done by physical or chemical methods. | Due to the strong bonds present between the molecules of a compound, separation can only be done by chemical methods. |
| Properties | The properties of an element are the same as the atoms of an element. | The properties of a compound are entirely different from the elements of which the compound is made. |
| Identification | Elements can be identified by their specific atomic numbers | Compounds are identified by their chemical formula, and bonds present between the atoms. |
| Smallest unit | An atom is the smallest particle of an element. | A molecule is considered to be the smallest particle of a compound. |
| Example | Oxygen, nitrogen, etc. | Sodium chloride, calcium carbonate. |
Difference Between Elements And Compounds Class 9th
What is the difference between an element and a compound?
Elements are products of the similar type of atoms. For example – O2 is oxygen which is formed with two oxygen atoms which are the same and have the same atomic number and properties.
Compounds are formed with two or more than two types of atoms. For example, H20. This is the chemical formula of water, which contains two hydrogen atoms and one oxygen atom. A covalent bond is present between H and O atoms, due to which water has different properties.
More Details Of Elements
An element consists of the same number of protons, meaning that the atoms in an element have the same atomic number. It is impossible to break down an element.
There can be certain differences in the number of neutrons, although the number of protons is the same. As a result, they can be of different masses.
More Details Of Compounds
When a chemical reaction occurs, multiple substances combine together for the formation of a compound. A compound behaves entirely differently from the elements that it consists of. For example in H2O, hydrogen and oxygena are gaseous at room temperature whereas H2O is a liquid at room temperature.
FAQs on Difference Between Element and Compound
What is the difference between an element and a compound? Write any one difference
The difference between an element and compound is that the representation of an element is by a symbol while a compound is represented by a chemical formula.
What are elements and compounds?
The smallest particle of an element is an atom and the smallest particle of a compound is a molecule.
How many elements and compounds are there?
There are 118 elements and an infinite number of compounds.
Is there a possibility that elements and compounds can be broken down?
Elements cannot be broken down with any chemical reaction. With the help of chemical reaction, compounds can be broken down and can be converted into new forms.
Write four examples of both elements and compounds.
Elements - N2, H2, He2, Cl2. Compounds - NaOH, CO,, C2H2, H2O2.








