Introduction to Hydrochloric Acid Formula
Hydrochloric acid (HCl) is a strong, highly corrosive acid commonly found in laboratories and industrial settings. It is a clear, colorless liquid with a pungent smell. Hydrochloric acid is composed of hydrogen (H) and chlorine (Cl) atoms, and its chemical formula is HCl.
Structural Formula: The structural formula of hydrochloric acid represents the arrangement of atoms within the molecule. In the case of HCl, it is a simple structure with a single covalent bond between the hydrogen atom (H) and the chlorine atom (Cl):
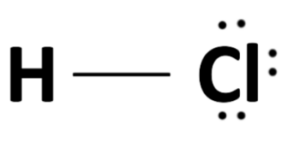
Hydrochloric acid is an important chemical compound with various uses and applications. It is classified as a strong acid because, when dissolved in water, it ionizes almost completely, releasing hydrogen ions (H+) into the solution.
In aqueous solutions, hydrochloric acid is highly reactive due to its acidity. It has a low pH and is corrosive to many materials, including metals. It is used in various industrial processes, such as metal cleaning and pickling, pH adjustment, and as a laboratory reagent.
In the human body, hydrochloric acid plays a crucial role in the stomach, where it aids in the digestion of food. It helps break down proteins into smaller peptides and amino acids, creating an acidic environment necessary for the activation of digestive enzymes.
Due to its corrosive nature, hydrochloric acid must be handled with caution and appropriate safety measures. Protective equipment, such as gloves and goggles, should be worn when working with or around hydrochloric acid. It should be stored in a well-ventilated area and kept away from incompatible substances.
It’s important to note that hydrochloric acid should be used only in controlled and appropriate settings by individuals with the necessary knowledge and expertise, as mishandling can result in serious injuries or damage to property.
Use and Application of Hydrochloric Acid:
- Industrial Cleaning: Hydrochloric acid is commonly used for industrial cleaning purposes, particularly in metal fabrication and processing industries. It is effective in removing rust, scale, and other deposits from metal surfaces, making it a useful ingredient in various cleaning solutions.
- pH Adjustment: Hydrochloric acid is used to adjust the pH of solutions in a wide range of industries. It is particularly useful in water treatment plants, swimming pools, and aquariums, where maintaining the appropriate pH level is important for the overall water quality and the well-being of aquatic life.
- Pickling: In metalworking, hydrochloric acid is used in the pickling process to remove oxides, scale, and other impurities from the surface of metals. This helps prepare the metal for further processing, such as welding, plating, or painting, by providing a clean and smooth surface.
- Laboratory Applications: Hydrochloric acid is a common reagent in laboratory settings. It is used for various purposes, including adjusting pH in chemical reactions, preparing solutions, and performing acid-base titrations. It is also utilized in qualitative and quantitative analysis of substances.
- Digestive Aid: Hydrochloric acid is naturally produced in the stomach to aid in the digestion of food. It helps break down proteins into smaller components, facilitating their absorption and digestion in the intestines. In certain medical conditions, hydrochloric acid supplements may be prescribed to improve digestion.
Solved examples on Hydrochloride acid Formula:
Example 1: Dilution Calculation You have 500 mL of a hydrochloric acid solution with a concentration of 2.0 M. How many milliliters of this solution should you use to prepare 1.0 L of a 0.5 M hydrochloric acid solution?
The dilution formula is given by:
C1V1 = C2V2
where: C1 = initial concentration
V1 = initial volume
C2 = final concentration
V2 = final volume
Given: C1 = 2.0 M
V1 = 500 mL
C2 = 0.5 M
V2 = 1000 mL
Using the dilution formula, we can calculate V1:
C1V1 = C2V2
(2.0 M)(500 mL) = (0.5 M)(1000 mL)
1000 M mL = 500 M mL
V1 = 500 mL
Therefore, you should use 500 mL of the 2.0 M hydrochloric acid solution to prepare 1.0 L of the 0.5 M hydrochloric acid solution.
Example 2: Neutralization Reaction Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H2O). If you mix 100 mL of 1.0 M hydrochloric acid with 100 mL of 1.0 M sodium hydroxide, what is the limiting reagent and the number of moles of the excess reagent?
The balanced chemical equation for the neutralization reaction is:
HCl + NaOH → NaCl + H2O
According to the stoichiometry of the balanced equation, 1 mole of hydrochloric acid reacts with 1 mole of sodium hydroxide.
To determine the limiting reagent, we compare the number of moles of each reagent used:
Moles of HCl = (Concentration of HCl × Volume of HCl)
= (1.0 M × 0.1 L) = 0.1 moles
Moles of NaOH = (Concentration of NaOH × Volume of NaOH)
= (1.0 M × 0.1 L) = 0.1 moles
Since the moles of both reagents are equal, neither reagent is limiting. Therefore, no reagent is in excess.
Therefore, in the given reaction, neither hydrochloric acid nor sodium hydroxide is the limiting reagent, and there are no excess moles of any reagent.
Frequently asked Questions on Hydrochloric acid Formula:
- Is hydrochloric acid dangerous?
Yes, hydrochloric acid is a highly corrosive and toxic substance. It can cause severe burns and is harmful if inhaled or ingested. It should be handled with extreme caution, and proper safety measures, including the use of protective equipment, should be followed.
- Where can I buy hydrochloric acid?
Hydrochloric acid is available for purchase from chemical suppliers, hardware stores, and industrial supply companies. It is important to ensure that you have the necessary knowledge and understanding of handling and using the acid safely before purchasing it.
- Can hydrochloric acid be used for cleaning at home?
Hydrochloric acid is not recommended for general cleaning purposes at home due to its corrosive nature. It can damage surfaces, including metals, tiles, and fabrics. It should be used only in controlled and appropriate settings, following safety guidelines.
- How should I handle hydrochloric acid safely?
When handling hydrochloric acid, it is essential to wear protective equipment such as gloves, goggles, and an apron to prevent direct contact with the acid. It should be stored in a well-ventilated area, away from incompatible substances, and handled in a well-ventilated space.
- Can hydrochloric acid be disposed of down the drain?
No, hydrochloric acid should not be disposed of down the drain or in regular trash. It is considered hazardous waste and should be disposed of according to local regulations and guidelines. Contact your local waste management authority for proper disposal methods.
- Can hydrochloric acid be mixed with other chemicals?
Mixing hydrochloric acid with other chemicals should be done with caution and only when the compatibility of the substances is known. Certain combinations can produce hazardous reactions, releasing toxic gases or causing explosions. Always refer to safety data sheets and consult experts when considering chemical mixtures.




