Hydrogen Carbonate Formula
The hydrogen carbonate ion, also known as the bicarbonate ion, has the chemical formula HCO₃⁻. It consists of one hydrogen atom (H), one carbon atom (C), and three oxygen atoms (O), with a negative charge on the ion.
The formula of Hydrogen Carbonate
HCO₃⁻
It represents the hydrogen carbonate ion, also known as the bicarbonate ion. The ion consists of one hydrogen atom (H), one carbon atom (C), and three oxygen atoms (O). The negative charge (-) on the ion indicates that it has gained one extra electron, giving it a total of four oxygen atoms.
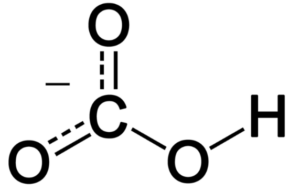
Structure of Hydrogen Carbonate
The hydrogen atom (H) is bonded to the carbon atom (C), and the carbon atom is bonded to three oxygen atoms (O). One of the oxygen atoms has a negative charge, indicating that it carries an extra electron and has a formal charge of -1. This negative charge makes the ion overall negatively charged.
The hydrogen carbonate ion is a polyatomic ion and is commonly found in compounds such as sodium bicarbonate (NaHCO₃) and potassium bicarbonate (KHCO₃). It plays important roles in biological and chemical systems, including maintaining the pH balance and participating in various acid-base reactions.
The structure of the hydrogen carbonate ion can be visualized as follows:
Physical properties of the Hydrogen Carbonate:
– State: The hydrogen carbonate ion does not exist as a standalone compound but is found in the form of salts or in solution.
– Solubility: Hydrogen carbonate salts are generally soluble in water, and the bicarbonate ion can readily dissolve in aqueous solutions.
Chemical properties of the Hydrogen Carbonate:
– Acid-Base Properties: The hydrogen carbonate ion acts as a weak acid in water, releasing a hydrogen ion (H⁺) to form carbonic acid (H₂CO₃). It can also act as a base by accepting a proton to form carbonate ions (CO₃²⁻).
– Buffering Capacity: Hydrogen carbonate ions play a crucial role in maintaining the pH balance in biological systems. They act as buffers, helping to resist changes in pH by neutralizing acids or bases.
– Decomposition: When heated or in the presence of an acid, hydrogen carbonate compounds decompose to release carbon dioxide gas (CO₂), water (H₂O), and a salt. This reaction is commonly observed in baking soda during cooking or in the fizzing of antacid tablets.
– Reaction with Acids: Hydrogen carbonate ions can react with acids to form carbon dioxide gas, water, and a salt.
Solved Example on the Hydrogen Carbonate formula (HCO₃⁻)
Example 1: Calculate the molar mass of the hydrogen carbonate ion (HCO₃⁻).
Solution:
The molar mass of hydrogen (H) is approximately 1 gram/mole.
The molar mass of carbon (C) is approximately 12 grams/mole.
The molar mass of oxygen (O) is approximately 16 grams/mole.
To calculate the molar mass of the hydrogen carbonate ion:
Molar mass = (1 × 1) + (1 × 12) + (3 × 16) = 1 + 12 + 48 = 61 grams/mole.
Example 2: Write the balanced chemical equation for the reaction between hydrochloric acid (HCl) and hydrogen carbonate (HCO₃⁻) to form carbon dioxide (CO₂), water (H₂O), and chloride ion (Cl⁻).
Solution:
The balanced chemical equation for the reaction is as follows:
2HCl + HCO₃⁻ → CO₂ + H₂O + 2Cl⁻
In this reaction, hydrochloric acid (HCl) reacts with hydrogen carbonate (HCO₃⁻) to produce carbon dioxide (CO₂), water (H₂O), and chloride ion (Cl⁻). The equation is balanced with two hydrochloric acid molecules reacting with one hydrogen carbonate ion to form one carbon dioxide molecule, one water molecule, and two chloride ions.
Frequently asked questions on Hydrogen Carbonate
1: What is the old name of hydrogen carbonate?
Answer: The old name of hydrogen carbonate is “bicarbonate.” The term “bicarbonate” is derived from the fact that the hydrogen carbonate ion (HCO₃⁻) consists of one hydrogen atom (H) and one carbonate ion (CO₃²⁻). In older literature and chemical nomenclature, the term “bicarbonate” was commonly used to refer to the hydrogen carbonate ion and its corresponding compounds. However, the term “hydrogen carbonate” is now more commonly used to describe this ion in modern chemistry.
2: Is hydrogen carbonate strong or weak base?
Answer: The hydrogen carbonate ion (HCO₃⁻) can act as a weak base in certain contexts. When dissolved in water, the hydrogen carbonate ion can accept a proton (H⁺) from water molecules, forming carbonic acid (H₂CO₃). Carbonic acid can further dissociate to release additional protons, leading to the formation of bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻) ions.
The basicity of the hydrogen carbonate ion is considered weak because it does not readily and completely accept protons. The equilibrium between carbonic acid and the hydrogen carbonate ion is shifted towards the side of the acid, making the hydrogen carbonate ion a weak base.
However, it’s important to note that the strength of a base can also depend on the specific reaction conditions and the comparison with other bases. While the hydrogen carbonate ion is relatively weak compared to strong bases like hydroxide ions (OH⁻), it can still exhibit basic properties in appropriate chemical reactions.
3: Is hydrogen carbonate acidic or basic?
Answer: The hydrogen carbonate ion (HCO₃⁻) can exhibit both acidic and basic properties, depending on the specific context.
– Acidic Nature: When the hydrogen carbonate ion is dissolved in water, it can act as a weak acid. It can donate a proton (H⁺) to water molecules, forming carbonic acid (H₂CO₃). The equilibrium between the hydrogen carbonate ion and carbonic acid is shifted towards the acid side, indicating its acidic nature.
– Basic Nature: On the other hand, in certain reactions or when in the presence of stronger acids, the hydrogen carbonate ion can act as a weak base. It can accept a proton (H⁺) from stronger acids, forming carbon dioxide (CO₂), water (H₂O), and a salt.
4: What is hydrogen carbonate used in?
Answer: Hydrogen carbonate, also known as bicarbonate, has various applications in different fields. Here are some common uses of hydrogen carbonate:
–Baking: Sodium bicarbonate (NaHCO₃), commonly known as baking soda, is widely used in baking. It reacts with acidic ingredients like vinegar or lemon juice to release carbon dioxide gas, causing dough or batter to rise and giving a light texture to baked goods.
–Antacid: Sodium bicarbonate is also used as an antacid to relieve symptoms of heartburn, indigestion, and acid reflux. It works by neutralizing excess stomach acid.
–Cleaning Agent: Baking soda is a versatile cleaning agent used for various household cleaning purposes. It can be used as a mild abrasive for scrubbing surfaces, removing stains, and deodorizing.
–Fire Extinguisher: Certain types of fire extinguishers utilize potassium bicarbonate (KHCO₃) or sodium bicarbonate as an effective agent for suppressing fires involving flammable liquids and electrical equipment. The bicarbonate releases carbon dioxide, which helps smother the fire.
–pH Buffer: Hydrogen carbonate is commonly used as a pH buffer in laboratory settings and in the formulation of various pharmaceuticals. It helps maintain the pH balance in solutions and prevents large fluctuations in acidity or alkalinity.
–Water Treatment: Bicarbonates, including hydrogen carbonate, are present in natural water sources. They contribute to the alkalinity of water and help buffer against pH changes. In water treatment processes, bicarbonates can be adjusted to optimize water chemistry and prevent corrosion in pipes and equipment.
–Carbonated Beverages: Hydrogen carbonate plays a role in the carbonation process of many beverages, including carbonated water, soft drinks, and sparkling wines. It helps create the fizz or effervescence by releasing carbon dioxide when the container is opened.
5: What is the chemical formula of hydrogen carbonate?
Answer: The chemical formula of hydrogen carbonate is HCO₃⁻.




