Hydrogen peroxide Formula
Hydrogen peroxide is a chemical compound with the formula H2O2. In this note, we will explore the formula and structure of hydrogen peroxide, as well as its chemical and physical properties.
Formula and Structure of Hydrogen Peroxide:
The formula for hydrogen peroxide is H2O2. It indicates that each molecule of hydrogen peroxide consists of two hydrogen (H) atoms and two oxygen (O) atoms. The structure of hydrogen peroxide is best represented as two hydroxyl (OH) groups connected by a single oxygen-oxygen bond.
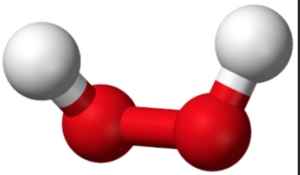
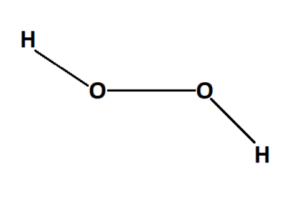
hydrogen peroxide (H2O2)
Chemical Properties of Hydrogen Peroxide:
- Oxidizing Agent: Hydrogen peroxide is a powerful oxidizing agent. It readily donates oxygen atoms, which can react with other substances, leading to oxidation reactions. It can oxidize various organic and inorganic compounds.
- Decomposition: Hydrogen peroxide is relatively unstable and can decompose into water (H2O) and oxygen (O2) over time. This process is accelerated by heat, light, or the presence of catalysts such as manganese dioxide (MnO2).
- Disinfectant and Bleaching Agent: Due to its ability to oxidize and break down organic compounds, hydrogen peroxide is commonly used as a disinfectant and bleaching agent. It is effective in killing bacteria, viruses, and fungi.
Physical Properties of Hydrogen Peroxide:
- Appearance: Hydrogen peroxide is a colorless liquid at room temperature. However, it may appear pale blue in a concentrated form.
- Solubility: Hydrogen peroxide is miscible in water, meaning it can dissolve in water in all proportions. The solubility of hydrogen peroxide decreases with decreasing temperature.
- Density: The density of hydrogen peroxide is approximately 1.45 g/cm³. However, the density can vary depending on the concentration of the solution.
- Boiling Point: Hydrogen peroxide has a boiling point of approximately 150.2°C (302.4°F). However, it can decompose before reaching its boiling point.
- Stability: Hydrogen peroxide is relatively unstable and can decompose slowly even at room temperature. It is often stored in a brown bottle to protect it from light, which can accelerate the decomposition process.
Solved Examples on Hydrogen peroxide Formula:
Example 1: Calculate the percent composition of hydrogen in hydrogen peroxide.
Solution:
To calculate the percent composition of hydrogen in hydrogen peroxide, we need to determine the molar mass of hydrogen and the molar mass of hydrogen peroxide, and then divide the molar mass of hydrogen by the molar mass of hydrogen peroxide, multiplied by 100.
The molar mass of hydrogen (H) = 1.008 g/mol
The molar mass of hydrogen peroxide (H2O2) = (2 * molar mass of hydrogen) + (2 * molar mass of oxygen)
= (2 * 1.008 g/mol) + (2 * 16.00 g/mol)
= 2.016 g/mol + 32.00 g/mol
= 34.016 g/mol
Percent composition of hydrogen = (Molar mass of hydrogen / Molar mass of hydrogen peroxide) * 100
= (2.016 g/mol / 34.016 g/mol) * 100
≈ 5.93%
Therefore, hydrogen constitutes approximately 5.93% of the total mass of hydrogen peroxide.
Example 2: Write the balanced chemical equation for the decomposition of hydrogen peroxide into water and oxygen gas.
Solution:
The decomposition reaction of hydrogen peroxide can be represented by the following balanced chemical equation:
2 H2O2 (aq) → 2 H2O (l) + O2 (g)
This equation indicates that two molecules of hydrogen peroxide decompose into two molecules of water and one molecule of oxygen gas. The (aq) notation denotes that hydrogen peroxide is dissolved in water, (l) denotes liquid water, and (g) denotes gaseous oxygen.
Frequently asked Questions on Hydrogen peroxide Formula:
1: What is the chemical formula of hydrogen peroxide?
Answer: The chemical formula of hydrogen peroxide is H2O2. It indicates that each molecule of hydrogen peroxide consists of two hydrogen (H) atoms and two oxygen (O) atoms.
2: Is hydrogen peroxide a stable compound?
Answer: Hydrogen peroxide is relatively unstable and can decompose over time into water and oxygen. The decomposition process is accelerated by factors such as heat, light, or the presence of catalysts.
3: What is the role of hydrogen peroxide in hair bleaching?
Answer: Hydrogen peroxide is commonly used in hair bleaching products. It acts as an oxidizing agent and helps to break down the natural pigments in the hair, lightening its color. The decomposition of hydrogen peroxide releases oxygen, which further aids in the oxidation process.
4: Can hydrogen peroxide be used as a disinfectant?
Answer: Yes, hydrogen peroxide is often used as a disinfectant. It has antimicrobial properties and can kill a wide range of bacteria, viruses, and fungi. Hydrogen peroxide acts by damaging the cells of microorganisms through oxidation.
5: What are the common uses of hydrogen peroxide?
Answer: Hydrogen peroxide has various uses in different industries and settings. It is used as a cleaning agent, disinfectant, and bleaching agent. It is also used in wastewater treatment, as an oxygen source for plants in hydroponics, and as a propellant in rocketry.




