Phosphoric Acid Formula
The formula for phosphoric acid is H₃PO₄, indicating a molecule composed of three hydrogen atoms (H), one phosphorus atom (P), and four oxygen atoms (O).
Formula Structure of Phosphoric Acid Formula
In phosphoric acid (H₃PO₄), the three hydrogen atoms are bonded to the oxygen atoms, and one oxygen atom is double-bonded to the phosphorus atom. The structure can be represented as follows:
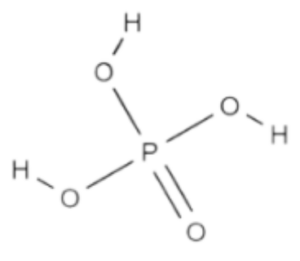
Phosphoric Acid (H₃PO₄)
Physical Properties of Phosphoric Acid
- State of Matter: Phosphoric acid is a colorless, viscous liquid.
- Density: The density of phosphoric acid is approximately 1.87 grams per cubic centimeter (g/cm³) at room temperature.
- Melting Point: Phosphoric acid has a relatively high melting point of about 42.35 degrees Celsius (108.23 degrees Fahrenheit).
- Boiling Point: Phosphoric acid has a boiling point of around 158 degrees Celsius (316 degrees Fahrenheit).
- Solubility: Phosphoric acid is highly soluble in water, forming a strongly acidic solution.
Chemical Properties of Phosphoric Acid Formula
- Acidic Nature: Phosphoric acid is a strong acid that readily donates protons (H⁺) when dissolved in water, making it an effective acid in various chemical reactions.
- Reactivity with Metals: Phosphoric acid can react with certain metals to produce hydrogen gas and soluble metal phosphate salts.
- Dehydration Reactions: Phosphoric acid can undergo dehydration reactions, losing water molecules to form polyphosphoric acids or condensed phosphates.
- Buffering Capacity: Phosphoric acid can act as a buffer due to its ability to accept or donate protons, helping to stabilize pH in a solution.
Solved examples on Phosphoric Acid Formula
Example 1: Reaction with Sodium Hydroxide
Answer: When phosphoric acid reacts with sodium hydroxide, a neutralization reaction occurs, resulting in the formation of sodium phosphate and water.
H₃PO₄ + 3 NaOH → Na₃PO₄ + 3 H₂O
In this reaction, the hydrogen ions from phosphoric acid combine with the hydroxide ions from sodium hydroxide to form water, while the remaining ions form sodium phosphate.
Example 2: Reaction with Calcium Carbonate
Answer: Phosphoric acid can react with calcium carbonate, a common compound found in rocks and minerals, resulting in the formation of calcium phosphate, carbon dioxide, and water.
2 H₃PO₄ + 3 CaCO₃ → Ca₃(PO₄)₂ + 3 CO₂ + 3 H₂O
In this reaction, the phosphoric acid reacts with calcium carbonate to produce calcium phosphate, carbon dioxide gas, and water.
Frequently asked questions on Phosphoric acid
1: What is the chemical formula of phosphoric acid?
Answer: The chemical formula of phosphoric acid, H₃PO₄, indicates the composition of the molecule. It consists of three hydrogen atoms (H), one phosphorus atom (P), and four oxygen atoms (O).
In the formula, the subscript numbers indicate the number of atoms present. The subscript 3 after hydrogen (H₃) means that there are three hydrogen atoms in the molecule. The phosphorus atom (P) is not followed by a subscript, indicating that there is only one phosphorus atom in the molecule. The subscript 4 after oxygen (O₄) signifies that there are four oxygen atoms in the molecule.
2: What is the basicity of phosphoric acid?
Answer: Phosphoric acid is a tribasic acid. It has three replaceable hydrogen atoms.
Phosphoric acid has three ionizable hydrogen atoms, which means it can donate up to three protons. Each deprotonation step results in the formation of a conjugate base:
- First deprotonation: H₃PO₄ → H₂PO₄⁻ + H⁺
The first hydrogen atom is donated, forming the dihydrogen phosphate ion (H₂PO₄⁻).
- Second deprotonation: H₂PO₄⁻ → HPO₄²⁻ + H⁺
The second hydrogen atom is donated, producing the monohydrogen phosphate ion (HPO₄²⁻).
- Third deprotonation: HPO₄²⁻ → PO₄³⁻ + H⁺
The third and final hydrogen atom is donated, resulting in the formation of the phosphate ion (PO₄³⁻).
3: What happens when phosphoric acid is exposed to high temperatures?
Answer: When phosphoric acid (H₃PO₄) is exposed to high temperatures, it undergoes various chemical reactions and transformations. The specific reactions and products formed depend on the temperature and conditions of the reaction. Here are a few notable changes that can occur:
- Dehydration: At high temperatures, phosphoric acid can undergo dehydration reactions, where it loses water molecules. The resulting compounds are polyphosphoric acids or condensed phosphates. For example, at temperatures around 180-200 degrees Celsius, orthophosphoric acid (H₃PO₄) can dehydrate to form pyrophosphoric acid (H₄P₂O₇).
- Decomposition: At very high temperatures, phosphoric acid can decompose into various products, including phosphorous oxides and water vapor. The specific decomposition products depend on the temperature range and reaction conditions. For example, at temperatures above 550 degrees Celsius, phosphoric acid can decompose into phosphorous pentoxide (P₂O₅) and water (H₂O).
4: What are the uses of phosphoric acid?
Answer: Phosphoric acid (H₃PO₄) has several important uses across various industries. Here are some notable applications:
- Food and Beverage Industry: Phosphoric acid is commonly used as an acidulant and pH regulator in the food and beverage industry. It is added to soft drinks, including colas, to provide a tangy flavor and act as a buffering agent to maintain a desired acidity level.
- Fertilizer Production: Phosphoric acid is a key ingredient in the production of phosphate-based fertilizers. It is used to convert phosphate rock into water-soluble phosphates, which can be easily taken up by plants. These fertilizers help enhance soil fertility and promote healthy plant growth.
- Water Treatment: Phosphoric acid is employed in water treatment processes to adjust pH levels and control the formation of scale and corrosion in pipes, boilers, and cooling systems. It can help prevent the deposition of calcium and magnesium salts, thereby ensuring efficient operation of water treatment systems.
- Detergent and Cleaning Products: Phosphoric acid is utilized in cleaning products, such as metal cleaners and rust removers, due to its ability to dissolve and remove mineral deposits, rust, and scale. It is also employed in some household cleaning solutions to remove hard water stains.
- Industrial Applications: Phosphoric acid finds use in various industrial processes. It is utilized in the production of phosphate-based chemicals, including flame retardants, detergent builders, and metal surface treatment chemicals. It also serves as a catalyst in certain chemical reactions and is employed in the synthesis of pharmaceuticals and other organic compounds.
- Dentistry: In dentistry, phosphoric acid is employed as an etching agent during dental procedures. It is used to prepare the tooth surface before applying dental adhesives or composite materials, enhancing their adhesion to the tooth structure.
5: What are phosphates? Give an example.
Answer: Phosphates are the salts of phosphoric acid. Phosphates are chemical compounds containing the phosphate ion (PO₄³⁻).
- They are composed of phosphorus (P) atoms bonded to four oxygen (O) atoms in a tetrahedral arrangement.
- Phosphates are essential in various biological and environmental processes, and they play a critical role in many industries and applications.
- In the food industry, sodium phosphate is employed as a food additive for several purposes. It acts as a pH regulator, buffering agent, and emulsifier in processed meats, cheese, and baked goods. Sodium phosphate is also used in the formulation of some sports drinks to replenish electrolytes lost during exercise.
- In medicine, sodium phosphate solutions are used as laxatives or for bowel cleansing before certain medical procedures. They help to induce bowel movements by increasing water content in the intestines, aiding in colon cleansing.
- Phosphates are a key nutrient in aquatic ecosystems, where they serve as a source of phosphorus for plants and algae.
- Phosphates are also used in detergents and cleaning products.
- They are essential for biological processes, contribute to the strength of bones and teeth, and have both beneficial and potentially harmful effects on ecosystems.
One example of a phosphate compound is sodium phosphate (Na₃PO₄). It consists of three sodium ions (Na⁺) and one phosphate ion (PO₄³⁻). Sodium phosphate is widely used in various fields, including food and beverage production, medicine, and laboratory research.




