Table of Contents
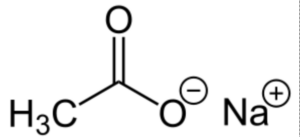
The formula for sodium acetate is CH₃COONa, which represents a compound containing one sodium ion (Na⁺) and one acetate ion (CH₃COO⁻).
Formula Structure of Sodium Acetate Formula
The molecular structure of sodium acetate consists of a sodium cation (Na⁺) bonded to the acetate anion (CH₃COO⁻). The acetate ion is composed of two carbon atoms (C), three hydrogen atoms (H), and two oxygen atoms (O).
Physical Properties of Sodium Acetate Formula:
- State of Matter: Sodium acetate is commonly found as a white crystalline solid.
- Solubility: It is highly soluble in water, forming a clear solution.
- Melting and Boiling Points: Sodium acetate has a melting point of 324 degrees Celsius (615 degrees Fahrenheit) and decomposes before reaching its boiling point.
- Odor: Sodium acetate is odorless.
Chemical Properties of Sodium Acetate Formula
- Buffering Capacity: Sodium acetate is a commonly used buffer in chemical and biological applications. It can help maintain a stable pH by resisting changes in acidity or alkalinity when small amounts of acid or base are added.
- Acetic Acid Source: Sodium acetate can act as a source of acetic acid. When dissolved in water, it can release acetic acid (CH₃COOH) ions, contributing to its acidic properties.
- Salt Formation: Sodium acetate can react with other acids to form acetate salts. For example, the reaction between sodium acetate and hydrochloric acid (HCl) results in the formation of sodium chloride (NaCl) and acetic acid.
- Heat-Release Reaction: Sodium acetate undergoes an interesting heat-release reaction called “hot ice” or “hand warmer.” When a supersaturated solution of sodium acetate is cooled below its melting point, it solidifies and releases heat upon crystallization.
Solved Examples of Sodium Acetate Formula
Example 1: Preparation of Sodium Acetate Solution
Solution:
Suppose you need to prepare a 0.1 M sodium acetate solution. To calculate the amount of sodium acetate needed, you can use its formula weight. The formula weight of sodium acetate is 82.03 g/mol.
Step 1: Determine the desired molarity (M) and volume (V) of the solution. Let’s say you want to prepare 500 mL of a 0.1 M sodium acetate solution.
Step 2: Calculate the number of moles of sodium acetate needed using the formula:
Moles = Molarity x Volume (in liters)
Moles = 0.1 mol/L x 0.5 L = 0.05 moles
Step 3: Convert moles to grams using the formula weight:
Grams = Moles x Formula Weight
Grams = 0.05 moles x 82.03 g/mol = 4.1015 g (approximately 4.1 g)
Step 4: Dissolve 4.1 g of sodium acetate in sufficient water to make 500 mL of solution. Stir until the solid is fully dissolved.
Example 2: Acid-Base Neutralization Reaction.
Solution:
Suppose you have a solution of hydrochloric acid (HCl) with a concentration of 0.05 M, and you want to neutralize it with sodium acetate solution. The balanced chemical equation for the reaction is
HCl + CH₃COONa → CH₃COOH + NaCl
Step 1: Determine the balanced equation.
Step 2: Calculate the amount of sodium acetate needed to react completely with the hydrochloric acid. Use stoichiometry and the molarity of HCl to find the moles of HCl.
Step 3: Use stoichiometry again to find the moles of sodium acetate required, knowing the molar ratio between HCl and sodium acetate.
Step 4: Prepare the sodium acetate solution with the calculated amount and slowly add it to the hydrochloric acid solution until neutralization is achieved.
Example 3: Crystallization of Sodium Acetate.
Solution:
Suppose you have a supersaturated solution of sodium acetate and you want to demonstrate its crystallization process.
Step 1: Heat a quantity of water and gradually add sodium acetate until no more solid can dissolve.
Step 2: Allow the solution to cool down slowly. As it cools, the sodium acetate will start to crystallize.
Step 3: To initiate crystallization, you can scratch the inside of the container or add a small crystal of sodium acetate as a seed.
Step 4: Observe the rapid crystallization of sodium acetate, releasing heat in the process. This phenomenon is known as “hot ice” or “hand warmer” due to the heat released during crystallization.
Frequently asked question of Sodium Acetate
1: What is sodium acetate used for?
Answer: In the textile industry, sodium acetate is used to neutralise waste sources of sulphuric acid and also as a photoresist using aniline dyes. It is also a pickling agent for chromium tanning and helps in the manufacture of synthetic rubber to avoid chloroprene vulcanisation.
2: Is sodium acetate a strong base?
Answer: Sodium acetate (CH3COONa) is a solid-state salt that can not be used in anhydrous or liquid form as an acid or base. Now, with NaOH being a strong base and CH3COOH being a weak acid, the resulting solution is fundamental in nature. Sodium acetate is therefore essential in an aqueous medium.
3: What is sodium acetate buffer?
Answer: Sodium acetate buffer solution in which an acetic acid maintains the pH — sodium acetate balance.
4: Why does sodium acetate release heat?
Answer: Once heated above 58oC, solid sodium acetate trihydrate loses its hydration capacity and starts to dissolve in that steam. Sodium acetate trihydrate solution heat is 19.7 kJ / mole (an endothermc process).
5: What is the common name for sodium acetate?
Answer: Sodium acetate is sodium salt of acetic acid. It has the C2H3O2Na chemical formula and is also known as sodium ethanoate.






