Sodium Bicarbonate Formula
Sodium bicarbonate, also known as baking soda, has the chemical formula NaHCO3. It is composed of one sodium ion (Na+), one hydrogen ion (H+), one carbon atom (C), and three oxygen atoms (O).
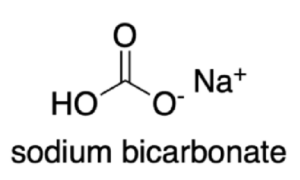
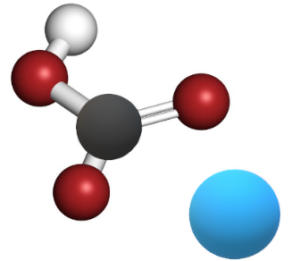
The molecular structure of sodium bicarbonate consists of a sodium cation (Na+) bonded to a bicarbonate anion (HCO3-). The bicarbonate anion is formed by one hydrogen ion bonded to a carbonate group (CO32-).
Physical properties of Sodium Bicarbonate:
- Appearance: Sodium bicarbonate is a white, crystalline powder or solid. It is commonly available in the form of fine granules.
- Solubility: It is highly soluble in water, meaning it dissolves readily to form a clear solution. This property makes it useful for various applications.
- Taste: Sodium bicarbonate has a slightly salty and alkaline taste.
- Melting Point: The melting point of sodium bicarbonate is around 50 degrees Celsius (122 degrees Fahrenheit). Upon heating, it decomposes and releases carbon dioxide gas.
Chemical properties of Sodium Bicarbonate:
- Acid-Base Properties: Sodium bicarbonate is amphoteric, meaning it can act as both an acid and a base. In water, it can dissociate and release bicarbonate ions, which can act as a weak base, and hydrogen ions, which can act as a weak acid.
- Decomposition: When heated, sodium bicarbonate decomposes into carbon dioxide gas (CO2), water (H2O), and sodium carbonate (Na2CO3). This reaction is commonly used in baking, where the release of carbon dioxide gas causes dough to rise.
- pH Regulation: Sodium bicarbonate can be used to adjust the pH of solutions. It acts as a buffering agent, helping to maintain a stable pH range. It can neutralize excess acid or base in a solution.
- Fire Extinguishing: Sodium bicarbonate can be used as a fire extinguisher, particularly for small fires involving flammable liquids or electrical equipment. When heated, it releases carbon dioxide, which displaces oxygen and suppresses the fire.
Sodium bicarbonate is widely used in various applications:
– Baking: It is a leavening agent in baking, causing dough to rise by producing carbon dioxide gas.
– Cooking: Sodium bicarbonate can be used for various culinary purposes, such as tenderizing meat or reducing acidity in dishes.
– Household Cleaning: It is effective in removing stains, odours, and grease. It can be used as a natural cleaning agent.
– Medical Applications: Sodium bicarbonate is used in antacid medications to relieve heartburn or indigestion. It is also used in some medical procedures, such as kidney dialysis.
– Personal Care: Sodium bicarbonate is found in toothpaste, deodorants, and bath products for its cleansing and odour-neutralizing properties.
Solved Examples on Sodium bicarbonate formula:
Example 1: How many oxygen atoms are present in one molecule of sodium bicarbonate?
Answer: The formula for sodium bicarbonate is NaHCO3. In one molecule of sodium bicarbonate, there are three oxygen atoms (O). This can be determined by counting the number of oxygen atoms present in the carbonate group (CO3) of the bicarbonate ion.
Example 2: What is the charge on the sodium ion in sodium bicarbonate?
Answer: In the formula NaHCO3, the sodium ion (Na+) has a charge of +1. This can be determined by considering the valence or oxidation states of the atoms in the compound. Hydrogen (H) typically has an oxidation state of +1, while oxygen (O) usually has an oxidation state of -2. Since there is one hydrogen atom in sodium bicarbonate, it has a total charge of +1 to balance the charge of the bicarbonate ion (HCO3-). Therefore, the sodium ion carries a charge of +1 to ensure electrical neutrality.
Frequently asked Questions on Sodium bicarbonate formula:
1: What is the chemical formula for sodium bicarbonate?
Answer: The chemical formula for sodium bicarbonate is NaHCO3. It indicates that each molecule of sodium bicarbonate consists of one sodium ion (Na+) bonded to one hydrogen ion (H+) and one bicarbonate ion (HCO3-).
2: What is the common name for sodium bicarbonate?
Answer: Sodium bicarbonate is commonly known as baking soda. It is widely used in baking and cooking as a leavening agent and for its ability to react with acids.
3: What is the purpose of sodium bicarbonate in baking?
Answer: Sodium bicarbonate serves as a leavening agent in baking. When combined with an acid (such as buttermilk or vinegar) and exposed to heat, it releases carbon dioxide gas, causing dough or batter to rise and create a light, fluffy texture in baked goods.
4: Is sodium bicarbonate the same as baking powder?
Answer: No, sodium bicarbonate and baking powder are not the same, although they are both used as leavening agents in baking. Baking powder is a mixture of sodium bicarbonate, an acid (such as cream of tartar), and a stabilizer (such as cornstarch). It provides a ready-to-use leavening action, as the acid is already combined with the sodium bicarbonate.
5: What are the uses of sodium bicarbonate?
Answer: Sodium bicarbonate has several uses beyond baking. It is commonly used as an antacid to relieve heartburn and indigestion. It is also used as a cleaning agent, deodorizer, and mild abrasive in household cleaning products. Additionally, sodium bicarbonate is utilized in medical treatments, such as intravenous administration to correct acid-base imbalances and in some medical tests.




