Table of Contents
Sodium Chloride Formula
Sodium chloride, commonly known as table salt, has the chemical formula NaCl. It is composed of one sodium ion (Na+) and one chloride ion (Cl–), which are held together by an ionic bond.
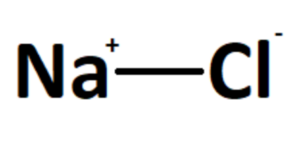
The molecular structure of sodium chloride can be represented as a repeating lattice of sodium and chloride ions. Each sodium ion is surrounded by six chloride ions, and each chloride ion is surrounded by six sodium ions. The structure forms a crystal lattice arrangement in which the positive and negative ions are strongly attracted to each other.
Physical properties of Sodium Chloride:
- Appearance: Sodium chloride is a white crystalline solid. It is commonly found in the form of small granules or fine powder.
- Taste: It has a salty taste, which is the characteristic flavor associated with salt.
- Solubility: Sodium chloride is highly soluble in water. It dissolves readily, forming a clear, colorless solution. The solubility of sodium chloride decreases with decreasing temperature.
- Melting and Boiling Points: Sodium chloride has a high melting point of 801 degrees Celsius (1474 degrees Fahrenheit) and a high boiling point of 1465 degrees Celsius (2669 degrees Fahrenheit).
Chemical properties of Sodium Chloride:
- Ionic Nature: Sodium chloride is an ionic compound, meaning it consists of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl–). These ions are held together by strong electrostatic forces.
- Dissociation: When sodium chloride is dissolved in water, it dissociates into sodium ions (Na+) and chloride ions (Cl–). This process is reversible, and the ions can recombine when the water evaporates, forming solid salt crystals.
- Conductivity: Sodium chloride is a good conductor of electricity when dissolved in water or in molten form. This is due to the presence of free-moving ions that can carry electric charge.
- Reactivity: Sodium chloride is chemically stable and does not readily react with most substances at normal conditions. However, it can react with certain reactive metals, such as sodium or potassium, to produce other compounds.
- Hygroscopicity: Sodium chloride has hygroscopic properties, meaning it can absorb moisture from the air, leading to clumping or caking if not stored properly.
Uses and Applications of Sodium chloride:
– Food Seasoning: It is the most common and widely used table salt for adding flavor to food.
– Food Preservation: Sodium chloride is used in the preservation of food, such as pickling and curing, as it inhibits the growth of bacteria and other microorganisms.
– Chemical Industry: It serves as a raw material for the production of various chemicals, including chlorine, caustic soda, and sodium carbonate.
– Water Treatment: Sodium chloride is used in water treatment processes, such as desalination and water softening.
– Medical Applications: It is used in saline solutions for medical purposes, such as intravenous fluid administration and wound irrigation.
Solved Examples on Sodium chloride Formula:
Example 1: What is the molar mass of sodium chloride (NaCl)?
Solution:
To calculate the molar mass of sodium chloride, we need to determine the atomic masses of sodium (Na) and chlorine (Cl) from the periodic table.
The atomic mass of sodium is approximately 22.99 g/mol, and the atomic mass of chlorine is approximately 35.45 g/mol.
Since there is one sodium atom and one chlorine atom in sodium chloride,
we can add their atomic masses together:
Molar mass of NaCl = (atomic mass of Na) + (atomic mass of Cl)
= 22.99 g/mol + 35.45 g/mol
= 58.44 g/mol
Therefore, the molar mass of sodium chloride is approximately 58.44 g/mol.
Example 2: How many moles of sodium chloride are present in 100 grams of NaCl?
Solution:
To calculate the number of moles of sodium chloride, we need to use the molar mass of NaCl, which is 58.44 g/mol.
We can use the formula:
Number of moles = Mass (in grams) / Molar mass
Number of moles = 100 g / 58.44 g/mol
≈ 1.71 mol
Therefore, there are approximately 1.71 moles of sodium chloride in 100 grams of NaCl.
Frequently asked Questions on sodium chloride formula:
1: What is the chemical formula for sodium chloride?
Answer: The chemical formula for sodium chloride is NaCl. It represents the combination of one sodium ion (Na+) and one chloride ion (Cl–) to form the compound.
2: What is the common name for sodium chloride?
Answer: Sodium chloride is commonly known as table salt. It is the most common salt used for seasoning and preserving food.
3: What is the crystal structure of sodium chloride?
Answer: Sodium chloride has a crystal lattice structure. It forms a repeating pattern of sodium and chloride ions held together by ionic bonds. This arrangement results in the formation of a cubic crystal structure.
4: Is sodium chloride soluble in water?
Answer: Yes, sodium chloride is highly soluble in water. It dissolves readily to form a clear, colorless solution. This property is one of the reasons why sodium chloride is widely used as table salt.
5: What are the uses of sodium chloride?
Answer: Sodium chloride has various uses beyond being a common table salt. It is used in food preservation, as a seasoning, and in the production of chlorine and caustic soda in the chemical industry. Sodium chloride is also used in water treatment processes, such as desalination, and in medical applications, such as saline solutions for intravenous fluids.






