Table of Contents
Toluene is an aromatic hydrocarbon with the chemical formula C7H8. Toluene is a clear, colourless liquid with a benzene-like odour. The chemical formula of toluene can be written as C6H5CH3. Toluene is a naturally occurring compound derived primarily from petroleum or petrochemical processes.
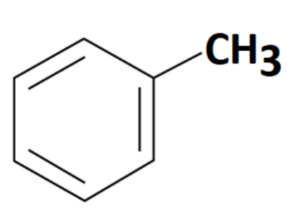
Formula and Structure of Toluene
The formula for toluene is C7H8, which indicates that it consists of seven carbon (C) atoms and eight hydrogen (H) atoms. Toluene is an aromatic compound, meaning it contains a benzene ring. It is a methyl-substituted derivative of benzene, with a methyl group (-CH3) attached to the benzene ring.
Chemical Properties of Toluene:
- Aromaticity: Toluene exhibits aromaticity due to the presence of a benzene ring. This aromatic nature is responsible for its unique chemical reactivity and stability.
- Solubility: Toluene is a nonpolar compound and is soluble in nonpolar organic solvents such as benzene, diethyl ether, and chloroform. It is relatively insoluble in water.
- Reactivity: Toluene is relatively stable under normal conditions. However, it can undergo various chemical reactions, including halogenation, nitration, sulfonation, and oxidation, due to the presence of the aromatic benzene ring.
- Combustibility: Toluene is highly flammable and can ignite easily. It has a low flashpoint and can form explosive mixtures with air. Proper safety precautions should be taken when handling and storing toluene.
Physical Properties of Toulene:
- Appearance: Toluene is a colorless liquid with a sweet, benzene-like odor. It has a characteristic aromatic smell.
- Density: The density of toluene is around 0.87 g/cm³. It is less dense than water, which has a density of 1 g/cm³.
- Boiling and Melting Points: Toluene has a boiling point of approximately 110.6°C (231.1°F) and a melting point of -95°C (-139°F). These values may vary depending on the purity of the toluene.
- Vapor Pressure: Toluene has a relatively high vapor pressure, meaning it readily evaporates at room temperature.
Applications of Toulene:
Toluene has several practical applications, including:
– Solvent: It is commonly used as a solvent in various industries, such as paints, coatings, adhesives, and chemical synthesis.
– Fuel: Toluene is used as a component in gasoline and aviation fuel.
– Production of Chemicals: It is a precursor in the production of other chemicals, including benzene, toluene diisocyanate (TDI), and xylenes.
– Pharmaceuticals: Toluene is used in the production of certain pharmaceutical drugs and as a solvent in drug formulation.
Solved Examples Related To The Formula Of Toluene (C7H8):
Example 1: Calculate the mass of toluene required to fill a container with a volume of 500 mL.
Solution:
To calculate the mass of toluene, we need to use its density and the given volume of the container.
Given:
Volume of the container = 500 mL = 0.5 L (since 1 L = 1000 mL)
Density of toluene = 0.87 g/cm³
Mass = Volume × Density
Mass = 0.5 L × 0.87 g/cm³ = 0.5 × 0.87 kg = 0.435 kg
Therefore, approximately 0.435 kg of toluene is required to fill a container with a volume of 500 mL.
Example 2: Calculate the number of moles of toluene in a 100 g sample.
Solution:
To calculate the number of moles, we need to divide the mass of the sample by the molar mass of toluene.
Given:
Mass of the sample = 100 g
Molar mass of toluene = 92.14 g/mol
Number of moles = Mass of the sample / Molar mass of toluene
Number of moles = 100 g / 92.14 g/mol
Number of moles ≈ 1.08 mol
Therefore, there are approximately 1.08 moles of toluene in a 100 g sample.
Frequently asked questions on Toluene Formula
1: Is toluene toxic or harmful to health?
Answer: Toluene can be harmful to health if inhaled, ingested, or absorbed through the skin in large amounts or over a prolonged period. It is known to have effects on the central nervous system and can cause symptoms like dizziness, headache, and nausea. Prolonged exposure to high concentrations may have more severe health effects.
2: What are the safety precautions to consider when working with toluene?
Answer: When working with toluene, it is important to ensure proper ventilation in the workspace to minimize inhalation. Personal protective equipment (PPE) such as gloves, goggles, and a lab coat should be worn. Avoid direct skin contact and ingestion. Additionally, storage and handling should comply with safety regulations and guidelines.
3:What are the common uses of toluene?
Answer: Toluene has various applications, including its use as a solvent in paints, coatings, adhesives, and cleaners. It is also used as a fuel additive and a component in the production of chemicals like benzene, xylenes, and toluene diisocyanate (TDI). Toluene is utilized in the synthesis of pharmaceuticals, dyes, and explosives as well.
4:Can toluene be found in everyday products?
Answer: Yes, toluene can be found in several everyday products. It is commonly present in paints, varnishes, thinners, and nail polishes. It can also be found in some cleaning agents, adhesives, and printing inks. Additionally, toluene can be present in cigarette smoke and gasoline.
5:Is toluene flammable?
Answer: Yes, toluene is highly flammable. It has a low flashpoint and can form explosive mixtures with air. It is important to take proper precautions to prevent ignition sources and handle toluene in well-ventilated areas away from open flames, sparks, and heat sources.






