Table of Contents
Ionization Enthalpy: Holding metals such as gallium and caesium in your hands causes them to melt owing to body heat. However, metals such as tungsten and gold have extremely high melting points. Have you ever wondered why this is so? Atoms are miniature replicas of our solar system, however, instead of planets, certain electrons and protons orbit the sun, which is the nucleus of the atoms. Assume you wish to remove an electron from an atom. However, the electron is clinging and stubborn, and it does not want to leave the atom. You should make a greater effort to remove that electron. The energy or force you use to accomplish this is measured in the ionization enthalpy.
Ionization enthalpy is a measure of an atom’s strength and how easily it may lose an electron. Many objects in the world operate using this notion, such as firecrackers and little circuits in electronic gadgets.
What is Ionization Enthalpy?
Ionization enthalpy is often referred to as Ionization energy. It is the total amount of energy necessary to extract an electron from an isolated gaseous atom. To put it simply, Ionization enthalpy is the least energy required by an atom to lose its outermost shell electrons. It also aids in understanding the behavioral pattern of atoms. Removing the electrons from the inner shells requires more energy than the outermost shells.
Ionization Enthalpy can be shown in the following equation:
Atom or ion(g) → Ion+ (g) + e-
Ionization The energy unit for enthalpy is kilojoule per mole (kJ/mol). Elements with low Ionization lose electrons more easily, forming positive ions. Elements having a high Ionization rate, on the other hand, acquire electrons quickly but produce negative ions.
Ionization Enthalpy Factors
Many factors affect the Ionization Enthalpy. The factors are as follows:
- Nucleus charge: Nucleus charge is proportional to ionization enthalpy. When there is more charge in the atom, more energy is required to remove the electron.
- Atomic size: The nucleus holds fewer outside electrons as the size of the atom increases. So it’s easy to remove the outermost electron.
- Penetration effect: The ability of the electron to get closer to the nucleus is known as the penetration effect.
- Shielding effect: It is an effect in which a shield is created by the innermost electrons so that the charge in the nucleus only remains for them and does not reach the outermost electrons.
- Electronic Configuration: The fully-filled orbitals and half-filled orbitals are stable. So, When you try to remove an electron from the fully-filled and half-filled it will require more energy. It will also make them less stable.
Bohr’s Atomic Model: Ionization Enthalpy
According to Bohr’s Atomic model, the electron can take a variety of pathways around the nucleus, which contains protons and neutrons. All of the pathways are at a given distance from the nucleus and reflect fixed energy.
Electrons absorb energy from their orbits. As they absorb energy, they go to the upper orbit, which has more energy. If the electron has absorbed a large quantity of energy, it is no longer attracted to the nucleus. It signifies the electron has left the atom.
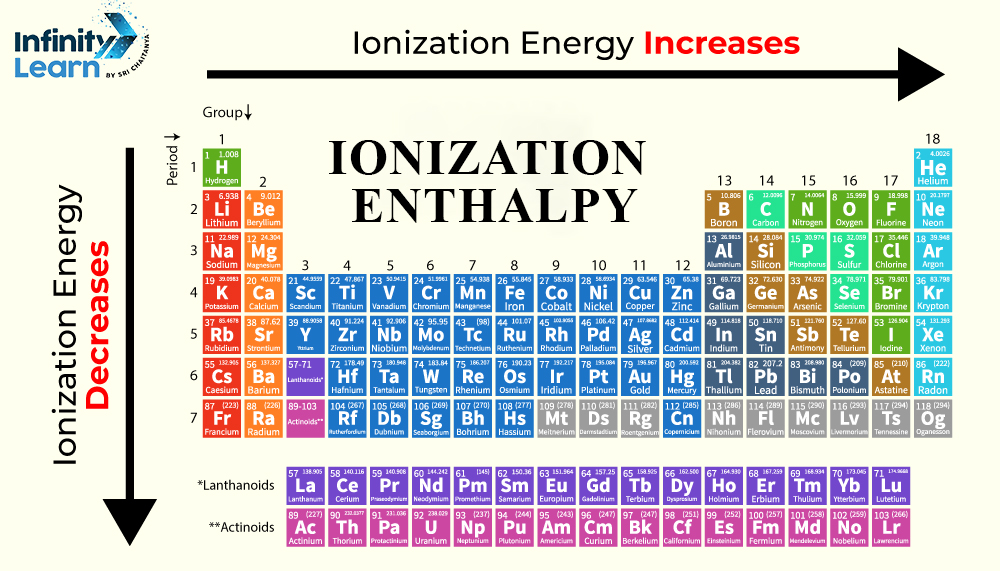
Ionization Enthalpy: Periodic Table Trends
Periods
An element’s atomic radius reduces as we move from left to right over a given period. When the atom is small, there is a strong attraction between the nucleus and the outermost shell of electrons. That is, the Ionization energy grows over time.
The second phase shows a difference in trend ionization enthalpy from Boron to Beryllium. Boron must have a higher Ionization enthalpy, but the converse is true. The primary reason for this is the penetration effect, and secondly, Beryllium is completely filled with shells.
Groups
The number of shells on the elements increases as we proceed down the group. This causes a decrease in ionization energy. The outermost shell will be farther away and less attracted to the charge of the nucleus. The shielding effect grows with the number of shells, whereas the ionization enthalpy eventually decreases.
Ionization Energy Formula
Ionization Enthalpy Definition: It means the minimum energy required by an atom to lose its outermost shell electrons. The energy for every ion shall be calculated in the periodic table. Let us understand the calculations used in measuring the energy:
The fundamental Ionization energy equation is;
Atom or ion(g) → Ion+ (g) + e-
As we remove the electron from the atom, the energy required to remove the other electron differs as compared to the first. Equation changes as per the change in the amount of energy required.
- First ionization energy equation
Atom or ion(g) → Ion+ (g) + e-
- Second ionization energy equation
Atom or ion(g) → Ion2+ (g) + e-
- Third ionization energy equation
Atom or ion(g)2 → Ion3+ (g) + e-
The Ionization energy tells how strongly the electron is attracted to the nucleus charge. The closer the electron to the nucleus, the more is the Ionization energy. But as the atomic radii increase the initiation energy decreases.
Measurement Principle of Ionization Enthalpy
The Ionization enthalpy of any element is determined in an electric discharge tube. Fast-moving electrons are produced in the tube as a result of electric currents and colliding with the atomic elements. The impact forces the electron to leave the atom.
On this page, you learned about the ionization enthalpy and how it operates. We saw the things that influence it as well as the underlying measuring premise. Remember, each element has a unique ionization enthalpy.
Valency
Valence describes the ability of an atom or a bonded group of atoms to connect with other atoms or atom groups. It depends on the electrons in the outermost layer (valence electrons) for an individual element. For groups of atoms with a charge, like SO4 2-, the valence is the charge itself.
Valency and its Periodic Trends
Elements are organized into groups (columns) on the periodic table based on the number of electrons they have in their outermost shell, known as valence electrons. The element’s position in the periodic table can tell us about its valence.
- In group 1, all elements have 1 valence electron, which makes their valence +1 because they tend to give away 1 electron.
- Similarly, in group 2, elements have 2 valence electrons and tend to give away 2 electrons, resulting in a valence of +2.
- Group 3 elements have 3 valence electrons and tend to give away 3 electrons, giving them a valence of +3.
- However, group 5 elements have 5 valence electrons, and they tend to take 3 electrons from other elements, leading to a valence of -3.
- Group 6 elements have 6 valence electrons, and they tend to take 2 electrons, resulting in a valence of -2.
- In the case of group 7 elements, which have 7 valence electrons, they tend to take 1 electron, leading to a valence of -1.
- Elements in group 8 do not react with other elements and have a valence of 0.
FAQs on Ionization Enthalpy
What do you mean by Ionization enthalpy?
The minimum energy required by an atom to lose its outermost shell electrons.
Which Element has the highest ionization energy?
Helium is the element that has the highest ionization energy among all the elements
Who has the lowest ionization energy?
Cesium is the element that has the lowest ionization energy among all the elements








